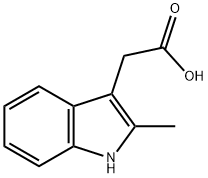
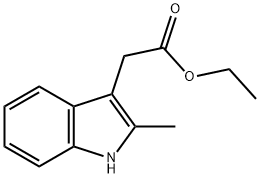
2-METHYLINDOLE-3-ACETIC ACID synthesis
- Product Name:2-METHYLINDOLE-3-ACETIC ACID
- CAS Number:1912-43-2
- Molecular formula:C11H11NO2
- Molecular Weight:189.21
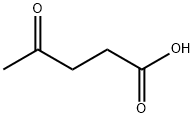
123-76-2
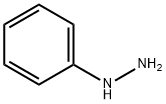
100-63-0

1912-43-2
GENERAL METHOD: Acetylpropionic acid (10 mmol) was dissolved in acetic acid (30 mL) and phenylhydrazine (15 mmol) was added to the solution under stirring conditions. The reaction mixture was heated to reflux for 4 hours. Upon completion of the reaction, it was cooled to room temperature and the mixture was poured into water (200 mL) and the pH was adjusted to 5-6 with 30% sodium hydroxide solution.The precipitated solid was collected by filtration, recrystallized from ethanol and dried under vacuum to give 2-methylindole-3-acetic acid (2a). 2-Methylindole-3-acetic acid (2a): yellow solid, 78% yield, melting point 200-203 °C (literature value 199-200 °C); 1H NMR (500 MHz, CDCl3) δ: 2.31 (3H, s, CH3), 3.65 (2H, s, CH2), 6.97 (1H, t, J = 7.5 Hz, ArH), 7.12 ( 1H, t, J = 7.5 Hz, ArH), 7.23 (1H, d, J = 6.0 Hz, ArH), 7.33 (1H, d, J = 6.0 Hz, ArH), 10.83 (1H, s, COOH), 12.15 (1H, s, NH); IR (νmax, cm-1): 1504, 1518, 1565, 1624, 1769, 2935, 3001, 3535; HRMS m/z: 190.0824 (calculated value C11H11NO2 [M + H]+: 190.0823).
Yield: 78%
Reaction Conditions:
with acetic acid for 4 h;Reflux;
Steps:
Preparation of compound 2
General procedure: Compound 1 (10 mmol) was dissolved in acetic acid (30 mL), andlevulinic acid (15 mmol) was added to the stirred solution which wasthen refluxed for 4 h. After cooling to room temperature, the mixturewas poured into water (200 mL) and then the solution was treated with30% sodium hydroxide to slight acidity (pH = 5-6). The precipitatedsolid was filtered off, recrystallised from ethanol and dried in vacuo togive compound 2.2-Methylindole-3-acetic acid (2a): Yellow solid, yield 78%, m.p.200-203 °C (199-200 °C14); 1H NMR (500 MHz, CDCl3), δ 2.31 (3H,s, CH3), 3.65 (2H, s, CH2), 6.97 (1H, t, J = 7.5 Hz, ArH), 7.12 (1H, t,J = 7.5 Hz, ArH), 7.23 (1H, d, J = 6.0 Hz, ArH), 7.33 (1H, d, J =6.0 Hz,ArH), 10.83 (1H, s, COOH), 12.15 (1H, s, NH); IR (νmax cm-1): 1504,1518, 1565, 1624, 1769, 2935, 3001, 3535; HRMS m/z 190.0824(calcd 190.0823 for C11H11NO2 [M + H]+)
References:
Wang, Xinying;Liu, Yizhou;Xu, Juan;Jiang, Fanwei;Kang, Congmin [Journal of Chemical Research,2016,vol. 40,# 10,p. 588 - 590]
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1912-43-2
116 suppliers
$10.00/1g
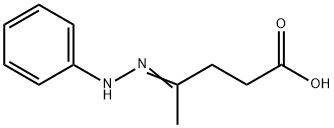
588-60-3
17 suppliers
$66.00/1g

1912-43-2
116 suppliers
$10.00/1g

123-76-2
501 suppliers
$5.00/25g

1912-43-2
116 suppliers
$10.00/1g
21909-49-9
29 suppliers
$75.29/1g

1912-43-2
116 suppliers
$10.00/1g