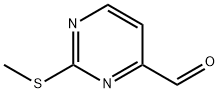
2-METHYLSULFANYL-PYRIMIDINE-4-CARBALDEHYDE synthesis
- Product Name:2-METHYLSULFANYL-PYRIMIDINE-4-CARBALDEHYDE
- CAS Number:1074-68-6
- Molecular formula:C6H6N2OS
- Molecular Weight:154.19
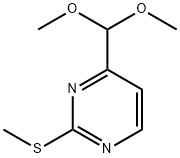
180869-36-7

1074-68-6
Example 4 Synthesis of 2-(methylthio)pyrimidine-4-carboxaldehyde (4): 4-(dimethoxymethyl)-2-(methylthio)pyrimidine (3) (53.7 g, 268 mmol) was slowly added to an aqueous solution of 1.2N HCl (300 mL, 268 mmol, 1.0 eq.) at 60 °C with continuous stirring for 3 hours. Upon completion of the reaction, the mixture was cooled to room temperature and neutralized by slow addition of solid sodium bicarbonate. Subsequently, the crude product was extracted with ether (3 x 150 mL) and the combined organic layers were concentrated under reduced pressure to afford the target product 2-(methylthio)pyrimidine-4-carboxaldehyde (4) as a yellow solid (14.2 g, 34% yield).LC-MS m/z 155 (M+1). Reference: WO 2006/009734 A1, p. 67.
497-19-8
1488 suppliers
$13.00/300G

180869-36-7
77 suppliers
$32.00/1g

1074-68-6
148 suppliers
$20.00/100mg
Yield: 7.49 g (97%)
Reaction Conditions:
with hydrogenchloride in ethyl acetate
Steps:
1.b b
b 2-Methylthiopyrimidine-4-carboxaldehyde The product of example 1(a) (9.96 g, 50 mmol), and 3 N HCl (42 mL, 126 mmol) were combined and stirred at 48° C. for 16 h, cooled to 23° C., combined with EtOAc (200 mL) and made basic by the addition of solid Na2 CO3 (12.6 g, 150 mmol). The aqueous phase was extracted with EtOAc (4*150 mL, dried (Na2 SO4), concentrated and the residue was filtered through a pad of silica (ca 150 mL) with CH2 Cl2 to afford 7.49 g (97%) of the title compound 1 H NMR (CDCl3): δ9.96 (s,1), 8.77 (d, 1), 7.44 (d, 1), 2.62 (s,3).
References:
SmithKline Beecham Corporation US5977103, 1999, A

180869-36-7
77 suppliers
$32.00/1g

1074-68-6
148 suppliers
$20.00/100mg
6342-56-9
323 suppliers
$8.00/25g

180869-36-7
77 suppliers
$32.00/1g

74-88-4
356 suppliers
$15.00/10g

1074-68-6
148 suppliers
$20.00/100mg