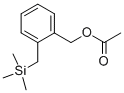
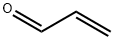2-Naphthalenecarboxaldehyde, 1,2,3,4-tetrahydro- synthesis
- Product Name:2-Naphthalenecarboxaldehyde, 1,2,3,4-tetrahydro-
- CAS Number:81292-68-4
- Molecular formula:C11H12O
- Molecular Weight:160.21

6947-15-5
9 suppliers
inquiry

81292-68-4
8 suppliers
inquiry
Yield:81292-68-4 44 mg
Reaction Conditions:
with Dess-Martin periodane in dichloromethane at 0; for 2 h;
Steps:
27 (2R,3R,4R,5S)-2-methyl-1-((1,2,3,4-tetrahydronaphthalen-2-yl)methyl)piperidine-3,4,5- triol
To a solution of 1,2,3,4-tetrahydro-2-naphthoic acid (120 mg, 0.68 mmol) in anhydrous THF (5 mL) at 0 °C, was added LAH (78 mg, 2.04 mmol), and the mixture was stirred at 0 °C for 2 h. The mixture was quenched slowly with satd. aqueous Na2SO4 and filtered. The solid was washed with EtOAc. The combined organic layer was washed with H2O (2 × 20 mL), separated, and dried over Na2SO4. After filtration, the solvent was evaporated under reduced pressure affording the crude (1,2,3,4-tetrahydronaphthalen-2- yl)methanol as a clear oil. To a solution of the obtained oil in anhydrous DCM (5 mL) at 0 °C, was added DMP (375 mg, 0.88 mmol), and the mixture was stirred at 0 °C for 2 h. The mixture was quenched slowly with Na2S3O5 solution. The mixture was extracted with DCM (3 × 20 mL). The combined organic layer was washed with H2O (2 × 10 mL), separated, and dried over Na2SO4. After filtration, the solvent was evaporated under reduced pressure, and the residue was purified on silica gel by flash chromatography affording 1,2,3,4- tetrahydronaphthalene-2-carbaldehyde as an oil (44 mg, 40%).
References:
WO2020/229968,2020,A1 Location in patent:Paragraph 00196

39246-30-5
2 suppliers
inquiry

81292-68-4
8 suppliers
inquiry
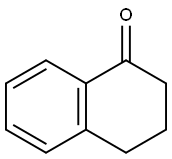
529-34-0
478 suppliers
$6.00/10g

81292-68-4
8 suppliers
inquiry

40685-04-9
0 suppliers
inquiry

81292-68-4
8 suppliers
inquiry