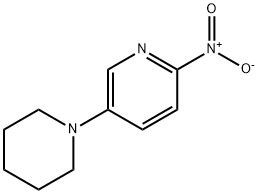
2-Nitro-5-(piperidin-1-yl)pyridine synthesis
- Product Name:2-Nitro-5-(piperidin-1-yl)pyridine
- CAS Number:444146-17-2
- Molecular formula:C10H13N3O2
- Molecular Weight:207.23
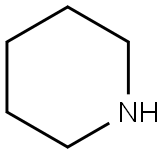
110-89-4
0 suppliers
$15.96/100ML
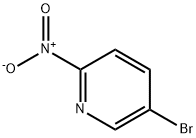
39856-50-3
544 suppliers
$6.00/5g
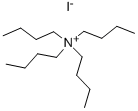
311-28-4
572 suppliers
$5.00/5g

444146-17-2
7 suppliers
inquiry
Yield:444146-17-2 85.7%
Reaction Conditions:
with potassium carbonate in dimethyl sulfoxide;
Steps:
101 6'-Nitro-3,4,5,6-tetrahydro-2H-[1,3']bipyridinyl
EXAMPLE 101 6'-Nitro-3,4,5,6-tetrahydro-2H-[1,3']bipyridinyl 5-Bromo-2-nitropyridine (5.6 g, 27.6 mmol), tetra-n-butyl ammonium iodide (0.510 g, 1.38 mmol), piperidine (2.58 g, 30.3 mmol) and potassium carbonate (3.85 g, 30.3 mmol) were mixed in DMSO (50 mL). The reaction mixture was warmed to 80° C. for 4 hours. The reaction mixture was diluted with ethyl acetate and filtered. The volume was reduced to remove ethyl acetate, the remaining solution was diluted with water (50 mL). A precipitate immediately formed and was collected by filtration and washed on the funnel with water to provide 6'-nitro-3,4,5,6-tetrahydro-2H-[1,3']bipyridinyl as an orange-brown solid (4.90 g, 85.7%). 1H NMR δ (400 MHz, CDCl3) 7.76 (s, 1H), 7.15 (d, J=7.3 Hz, 1H), 6.49 (d, J=8.5 Hz, 1H), 3.84 (m, 5H), 3.00 (m, 4H), 2.60 (s, 1H).
References:
US2003/149001,2003,A1