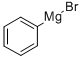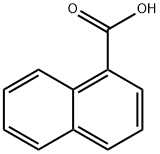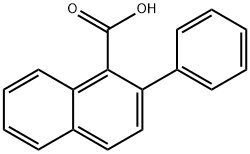
2-Phenylnaphthalene-1-carboxylic acid synthesis
- Product Name:2-Phenylnaphthalene-1-carboxylic acid
- CAS Number:108981-94-8
- Molecular formula:C17H12O2
- Molecular Weight:248.28
Yield:108981-94-8 68%
Reaction Conditions:
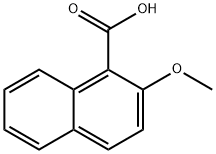
Stage #1: 2-methoxy-1-naphthoic acid;phenylmagnesium bromide in tetrahydrofuran; for 2 h;Inert atmosphere;Reflux;
Stage #2: with hydrogenchloride in tetrahydrofuran;water at 20; for 1 h;
Steps:
2 2-phenyl-naphthalene-1-carboxylic acid
2-phenyl-naphthalene-1-carboxylic acid Phenylmagnesium bromide (0.20 M in THF; 33.0 mL; 6.6 mmol) is added dropwise to a solution of 2-methoxynaphthalene-1-carboxylic acid (606 mg, 3.0 mmol) in 20 mL of anhydrous THF. The reaction mixture is refluxed for two hours, and then hydrolyzed at room temperature with distilled water (20 mL), acidified to pH=1 with an aqueous HCl solution (2M) and extracted with ethyl acetate (3*40 mL). The combined organic phases are dried over MgSO4, filtered then concentrated under reduced pressure. After recrystallization (cyclohexane/ethyl acetate: 1/3), 2-phenyl-naphthalene-1-carboxylic acid is isolated as a white solid (506 mg, 68%). Mp=118-120° C. (Alaka, R.; Indian J. Chem. 1967, 5, 610. 114° C.). 1H NMR (400 MHz, DMSO-d6) d: 8.29 (d, J=7.8 Hz, 1H), 7.88-7.83 (m, 2H), 7.73 (d, J=6.6 Hz, 2H), 7.47-7.44 (m, 2H), 7.33-7.25 (m, 4H). IR (ATR, cm-1): 3049, 1693, 1463, 1333, 861, 759. HRMS m/z calculated for C17H13O2 ([M+H]+): 249.0916 found 249.0940.
References:
US2012/316337,2012,A1 Location in patent:Page/Page column 22
591-50-4
500 suppliers
$10.00/1g

108981-94-8
2 suppliers
inquiry