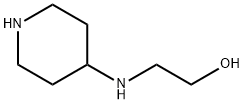
2-(piperidin-4-ylamino)ethanol synthesis
- Product Name:2-(piperidin-4-ylamino)ethanol
- CAS Number:875229-91-7
- Molecular formula:C7H16N2O
- Molecular Weight:144.21
![tert-butyl 4-[(2-hydroxyethyl)aMino]piperidine-1-carboxylate](/CAS/20150408/GIF/701298-37-5.gif)
701298-37-5
14 suppliers
$300.00/1g

875229-91-7
3 suppliers
inquiry
Yield:875229-91-7 95%
Reaction Conditions:
Stage #1: tert-butyl 4-[(2-hydroxyethyl)amino]piperidine-1-carboxylatewith trifluoroacetic acid
Stage #2: with hydrogenchloride in methanol at 20; for 0.5 h;
Stage #3: with sodium hydroxide in water at 20; pH=> 10; for 0.25 h;Cooling with ice;
Steps:
8
The 4-(2-ethanolamino)-1-Boc-piperidine (14g, 57.3mmol) was treated with TFA (28mL) ? for 90mL. The TFA was removed (reduced pressure) and the TFA salt was converted to the HCI salt by treatment with 20% HCl/methanol (140mL, 280mmol HCI) at ambient temperature for 30min. The HCl/methanol was evaporated (reduced pressure) to yield a white solid in quantitative yield. 19F NMR (MeOD) confirmed complete conversion to the HCI salt.The salt was dissolved in H20 (10mL) and cooled in an ice-bath. NaOH (10M, 35mL) was added to pH >10. The solution was warmed to RT and stirred for 15min prior to evaporating to dryness. The residue was triturated with ether/isopropanol(2:1), filtered and evaporated to dryness. The trituration process was repeated until no salt was present in the oil (5x) to yield 7.8g (95%) of a clear oil which solidified on standing.
References:
WO2012/142668,2012,A1 Location in patent:Page/Page column 51
41979-39-9
0 suppliers
$15.00/10mg

875229-91-7
3 suppliers
inquiry