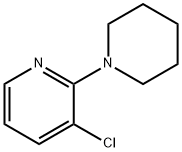
2-Piperidino-3-chloropyridine synthesis
- Product Name:2-Piperidino-3-chloropyridine
- CAS Number:282723-20-0
- Molecular formula:C10H13ClN2
- Molecular Weight:196.68
Yield:282723-20-0 76%
Reaction Conditions:
in toluene at 120; for 16 h;Sealed tube;
Steps:
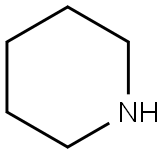
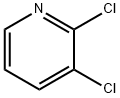
3-Chloro-2-(piperidin-1-yl)pyridine (4c)
General procedure: 2,3-Dichloropyridine (2.96 g, 20 mmol), piperidine (6.00 mL, 60mmol, 3.0 equiv), and toluene (20 mL) were mixed. Then, the reactionmixture was refluxed at 120 °C for 16 h in a sealed tube. After cooling,the mixture was quenched with H2O, extracted with DCM, and thecombined DCM layers were dried (Na2SO4). After filtration and evaporationof the solvent in vacuo, the crude product was purified by flashchromatography (n-pentane:EtOAc 8:2) to give 4c as a yellow liquid;yield: 2.99 g (15.2 mmol, 76%).1H NMR (400 MHz, CDCl3): = 8.16 (dd, J = 4.8, 1.7 Hz, 1 H), 7.55 (dd,J = 7.7, 1.7 Hz, 1 H), 6.78 (dd, J = 7.7, 4.8 Hz, 1 H), 3.34-3.19 (m, 4 H),1.80-1.65 (m, 4 H), 1.61 (td, J = 7.0, 3.6 Hz, 2 H).13C NMR (101 MHz, CDCl3): = 159.5, 145.7, 138.7, 123.0, 117.4, 50.5(2 C), 26.0 (2 C), 24.5
References:
Heinz, Benjamin;Djukanovic, Dimitrije;Siemens, Fiona;Idriess, Mohamed;Martin, Benjamin;Knochel, Paul [Synthesis,2021,vol. 54,# 22,p. 5055 - 5063]