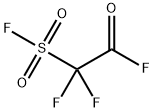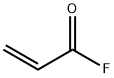
2-Propenoyl fluoride (9CI) synthesis
- Product Name:2-Propenoyl fluoride (9CI)
- CAS Number:430-72-8
- Molecular formula:C3H3FO
- Molecular Weight:74.05
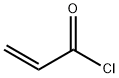
814-68-6
397 suppliers
$21.21/5gm:

430-72-8
0 suppliers
inquiry
Yield:-
Reaction Conditions:
with silver(II) fluoride at 20;Cooling with liquid nitrogen;
Steps:
2.1. Synthesis
ACRF was prepared by reaction of silver difluoride (Aldrich) andacryloyl chloride (Aldrich).Rigorously dry silver difluoride is essential for this chemistry. A 3-mol excess of silver difluoride was placed in a bulb containing a fewshort sections of Teflon tubing to aid mixing, and the contents wereheat gunned vigorously followed by pumping until with repetition the pressure rose only a fewmicrons of mercury. Acryloyl chloridewas condensedon the silver difluoride at liquid nitrogen temperature. Themixturewas warmed to room temperature and shaken for a few minutes.The product was distilled into another vessel, where a yellow color inthe cold condensate indicated that some elementary chlorine hadformed. Chlorinewas removed by distillation through a tube containinga 14-cm packing of copper metal turnings. A gas-phase IR spectrumshowed that the conversion from chloride to fluoride was incomplete[64,80]. To complete the reaction the sample was distilled twice througha column containing rigorously dry silver difluoride dispersed on glasswool and spaced with glass wool. Conversion of the chloride into thefluoridewas complete, and no chlorinewas formed. Very volatile silicontetrafluoride was a byproduct. It was removed from samples for spectroscopyby partial vaporization during slow warm-up from liquid nitrogentemperature. The vacuumsystemwasmade of borosilicate glass.
References:
Krasnoshchekov, Sergey V.;Craig, Norman C.;Koroleva, Lidiya A.;Stepanov, Nikolay F. [Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,2018,vol. 189,p. 66 - 79]
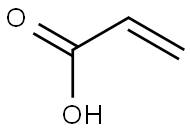
79-10-7
728 suppliers
$20.50/500ml

430-72-8
0 suppliers
inquiry