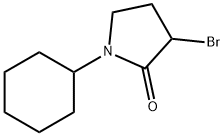
2-Pyrrolidinone, 3-bromo-1-cyclohexyl- synthesis
- Product Name:2-Pyrrolidinone, 3-bromo-1-cyclohexyl-
- CAS Number:852287-61-7
- Molecular formula:C10H16BrNO
- Molecular Weight:246.14
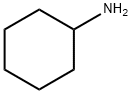
108-91-8
631 suppliers
$10.00/5mL
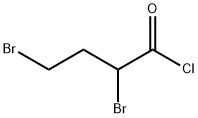
82820-87-9
63 suppliers
$35.35/250mgs:

852287-61-7
3 suppliers
inquiry
Yield:852287-61-7 34%
Reaction Conditions:
Stage #1: cyclohexylamine;2,4-dibromobutanoyl chloridewith sodium hydroxide in chloroform;water at 0 - 10; for 1 h;
Stage #2: with sodium hydride in tetrahydrofuran at 0; for 120 h;
Steps:
1
Preparation 1; 3-Bromo-l-cyclohexyl-pyrrolidin-2-one; EPO
References:
WO2006/49952,2006,A1 Location in patent:Page/Page column 49-50
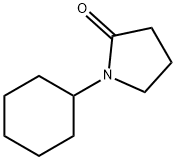
6837-24-7
191 suppliers
$19.00/25mL

852287-61-7
3 suppliers
inquiry