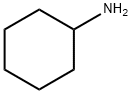
Cyclohexylamine
- CAS No.
- 108-91-8
- Chemical Name:
- Cyclohexylamine
- Synonyms
- CHA;Cyclohexylamin;AMINOCYCLOHEXANE;HEXAHYDROANILINE;cyclohexaneamine;CHA-60;cha[qr];AURORA KA-7609;CYCLOHEXYLAMINE;hexahydro-anilin
- CBNumber:
- CB8139274
- Molecular Formula:
- C6H13N
- Molecular Weight:
- 99.17
- MDL Number:
- MFCD00001486
- MOL File:
- 108-91-8.mol
- MSDS File:
- SDS
| Melting point | -17 °C |
|---|---|
| Boiling point | 134 °C(lit.) |
| Density | 0.867 g/mL at 25 °C(lit.) |
| vapor density | 3.42 (vs air) |
| vapor pressure | 10 mm Hg ( 22 °C) |
| refractive index |
n |
| Flash point | 90 °F |
| storage temp. | Store below +30°C. |
| solubility | organic solvents: miscible |
| form | Liquid |
| pka | 10.66(at 24℃) |
| color | Clear |
| PH | 11.5 (100g/l, H2O, 20℃) |
| Odor | strong fishy odor |
| explosive limit | 1.6-9.4%(V) |
| Water Solubility | MISCIBLE |
| FreezingPoint | -17.7℃ |
| Sensitive | Air Sensitive |
| Merck | 14,2729 |
| BRN | 471175 |
| Exposure limits | TLV-TWA 10 ppm (~40 mg/m3) (ACGIH). |
| Dielectric constant | 5.3(-21℃) |
| InChIKey | PAFZNILMFXTMIY-UHFFFAOYSA-N |
| LogP | 3.7 at 25℃ |
| Substances Added to Food (formerly EAFUS) | CYCLOHEXYLAMINE |
| CAS DataBase Reference | 108-91-8(CAS DataBase Reference) |
| EWG's Food Scores | 2-5 |
| FDA UNII | I6GH4W7AEG |
| NIST Chemistry Reference | Cyclohexanamine(108-91-8) |
| EPA Substance Registry System | Cyclohexylamine (108-91-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS02,GHS05,GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H226-H301+H311-H302+H312-H314-H361fd | |||||||||
| Precautionary statements | P202-P210-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | C,T | |||||||||
| Risk Statements | 10-21/22-34-62-24-22 | |||||||||
| Safety Statements | 36/37/39-45-1/2-26 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 10 ppm (40 mg/m3) | |||||||||
| RIDADR | UN 2357 8/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | GX0700000 | |||||||||
| F | 10-34 | |||||||||
| Autoignition Temperature | 559 °F | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2921 30 10 | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| Toxicity | LD50 orally in rats: 0.71 ml/kg (Smyth) | |||||||||
| NFPA 704 |
|
Cyclohexylamine price More Price(26)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.02885 | Cyclohexylamine for synthesis | 108-91-8 | 100mL | $44.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.02885 | Cyclohexylamine for synthesis | 108-91-8 | 1L | $59.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.02885 | Cyclohexylamine for synthesis | 108-91-8 | 2.5L | $129 | 2024-03-01 | Buy |
| Sigma-Aldrich | 240648 | Cyclohexylamine ReagentPlus , ≥99.9% | 108-91-8 | 5ml | $37.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | 240648 | Cyclohexylamine ReagentPlus , ≥99.9% | 108-91-8 | 100ml | $38.5 | 2024-03-01 | Buy |
Cyclohexylamine Chemical Properties,Uses,Production
Description
Cyclohexylamine is a colorless to yellow liquid (amines, primary aromatic). It has an unpleasant fishyodor. Molecular weight=99.20; Specific gravity=0.87;Boiling point=134.4℃; Freezing/Melting point 52 -17.7℃; Vapor pressure=11 mmHg at 20℃; Flashpoint=31℃; Autoignition temperature=293℃. Explosivelimits: LEL=1.5%; UEL=9.4%. Hazard Identification(based on NFPA-704 M Rating System): Health 3,Flammability 3, Reactivity 0. Soluble in water.
Chemical Properties
Cyclohexylamine is a colorless to yellow liquid (amines, primary aromatic). It has an unpleasant fishy odor. Flammable. It is infinitely miscible with water and conventional organic solvents. With water it forms an azeotrope that contains 44.2 % cyclohexylamine and boils at 96.4℃. Cyclohexylamine can be volatilized with water vapor. It can absorb carbon dioxide in the air and form a white crystalline carbonate. Aqueous solution is alkaline. 0.01% concentration of aqueous solution pH = 10.5. Its vapor and air to form an explosive mixture.
Uses
Cyclohexylamine is used in the manufacture of a number of products, including plasticizers, drycleaning soaps, insecticides, and emulsifying agents. It is also used as a corrosion inhibitor and in organic synthesis.
Uses
In organic synthesis, manufacture of insecticides, plasticizers, corrosion inhibitors, rubber chemicals, dyestuffs, emulsifying agents, dry-cleaning soaps, acid gas absorbents.
Cyclohexylamine is used primarily as corrosion inhibitor and vulcanization accelerator. Alone or mixed with other compounds, it has an anticorrosive action, for example, when used as an additive in heating oil or in the operation of steam boilers.
Cyclohexylamine functions as a hardener for epoxy resins and as a catalyst for polyurethanes. Sodium cyclohexylsulfamate and calcium cyclohexylsulfamate (cyclamates) are important artificial sweeteners. In polyamide polymerizations, cyclohexylamine is employed as chain terminator to control the molecular mass.
Definition
ChEBI: Cyclohexylamine is a primary aliphatic amine consisting of cyclohexane carrying an amino substituent. It has a role as a human xenobiotic metabolite and a mouse metabolite. It is a conjugate base of a cyclohexylammonium.
Production Methods
Cyclohexylamine is produced by the reaction of ammonia and cyclohexanol at elevated temperature and pressure in the presence of a silica-alumina catalyst (SRI 1985). It is also prepared by a similar process of catalytic hydrogenation of aniline at elevated temperature and pressure. Fractionation of the product of this reaction yields CHA, aniline, and a high-boiling residue containing n-phenylcyclohexylamine and dicyclohexylamine (Carswell and Morrill 1937). In 1982, U.S. production was 4.54 metric tons and 739.3 metric tons were imported into the U.S. (SRI 1985).
Reactions
Cyclohexylamine reacts with chlorine to form N,N-dichlorocyclohexylamine. N-Cyclohexylidenecyclohexylamine reacts with chloramine to give 1-cyclohexyl-3,3-pentamethylenediaziridine, which can be hydrolyzed to give cyclohexylhydrazine [46]. Cyclohexylamine and formaldehyde together react with peracetic acid to give 2-cyclohexyloxaziridine. In addition to using alkyl halides, alkyl sulfates, or alkyl phosphates, cyclohexylamine can be alkylated with an alcohol in the presence of a catalyst, such as aluminum oxide, copper, nickel, cobalt, or platinum, or by the Leuckart – Wallach method.
General Description
Cyclohexylamine appears as a clear colorless to yellow liquid with an odor of ammonia. Flash point 90 °F. Irritates the eyes and respiratory system. Skin contact may cause burns. Less dense than water. Vapors heavier than air. Toxic oxides of nitrogen produced during combustion.
Air & Water Reactions
Highly flammable. Sensitive to air and light. Soluble in water.
Reactivity Profile
Cyclohexylamine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
Health Hazard
Cyclohexylamine is a severe irritant to theeyes, skin, and respiratory passage. Skincontact can produce burns and sensitization;contact of the pure liquid or its concentratedsolutions with the eyes may cause loss ofvision.
The acute oral and dermal toxicity ofcyclohexylamine was moderate in test sub jects. The toxic effects include nausea, vom iting, and degenerative changes in the brain,liver, and kidney. Inhalation of its vaporsat high concentrations may cause a narcoticeffect.
LD50 value, oral (rats): 156 mg/kg
LD50 value, skin (rabbits): 277 mg/klg
Cyclohexylamine may be mutagenic, thetest for which has so far given inconclusiveresults. Administration of this compoundin animals produced a reproductive effect,including embryotoxicity and a reductionin male fertility. Intraperitoneal injectionof the amine in rats caused a dose dependent increase in chromosomal breaks.Roberts and coworkers (1989) studied themetabolism and testicular toxicity of cyclohexylamine (a metabolite of cyclamate)in rats and mice. Chronic dietary administration of 400 mg/kg/day for 13 weeksshowed decrease in organ weigh, histological changes, and testicular atrophy in boththe Wistar and dark agouti DA rats, but to awidely varying extent, while mice exhibitedno evidence of testicular damage.
There is no evidence of carcinogenicityin animals or humans caused by cyclohexy lamine.
Fire Hazard
When heated to decomposition, Cyclohexylamine emits highly toxic fumes. Vapor may travel a considerable distance to source of ignition and flash back. Toxic oxides of nitrogen are produced during combustion. Nitric acid; reacts vigorously with oxiding materials. Stable, avoid physical damage, storage with oxidizing material.
Industrial uses
The primary use of cyclohexylamine is as a corrosion inhibitor in boiler water treatment and in oil field applications (HSDB 1989). It is also a chemical intermediate for rubber processing chemicals, dyes (acid blue 62, former use), cyclamate artificial sweeteners and herbicides and a processing agent for nylon fiber production (SRI 1985). Windholz et al (1983) reports its use in the manufacture of insecticides, plasticizers, emulsifying agents, dry-cleaning soaps, and acid gas absorbents.
Safety Profile
A poison by ingestion, skin contact, and intraperitoneal routes. Experimental teratogenic and reproductive effects. A severe human skin irritant. Can cause dermatitis and convulsions. Human mutation data reported. Questionable carcinogen. Flammable liquid. Dangerous fire hazard when exposed to heat, flame, or oxidizers. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits toxic fumes of NOx.
Synthesis
Prepared by catalytic hydrogenation of aniline at elevated temp and pressures. Fractionation of crude reaction product yields cyclohexylamine, unchanged aniline, and high-boiling residue containing n-phenylcyclohexylamine (cyclohexylaniline) and dicyclohexylamine.
Potential Exposure
CHA is used in making dyes, chemi- cals, dry cleaning chemicals; insecticides, plasticizers, rub- ber chemicals; and as a chemical intermediate in the production of cyclamate sweeteners. Used in water treat- ment and as a boiler feedwater additive. It is also used in rubber production to retard degradation.
First aid
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin,remove contaminated clothing and wash immediately withsoap and water. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart actionhas stopped. Transfer promptly to a medical facility. Whenthis chemical has been swallowed, get medical attention. Ifvictim is conscious, administer water or milk. Do not inducevomiting. Medical observation is recommended for 24-48 hafter breathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consider administering a corticosteroidspray.
Note to physician: Treat for methemoglobinemia.
Spectrophotometry may be required for precise determination of levels of methemoglobinemia in urine
Carcinogenicity
According to the International Agency for Research of Cancer (IARC) working group, there is no evidence that cyclohexylamine is teratogenic or carcinogenic.
Metabolism
Generally, cyclohexylamine is readily absorbed and rapidly excreted from the body. After administration to rats, cyclohexylamine appears in body tissues with the highest concentrations in the lungs, spleen, liver, adrenals, heart, gastrointes- tinal tract and kidneys (Estep and Wiegand 1967 as reported by Bopp et al 1986).
After oral administration (0.2 g/kg) to rabbits, cyclohexylamine gave rise to unchanged cyclohexylamine and 7V-hydroxycyclohexylamine in the urine (Elliott et al 1968). When [14C]-labelled cyclohexylamine was administered, 68% of the radioactivity was recovered in the urine after 60 h. A small amount (0.5%) was eliminated in the breath and 45% of the administered dose was shown to be excreted in the urine as unconjugated cyclohexylamine, 0.2% as JV-hydroxycyclohexylamine in conjugated form, and 2.5% as cyclohexanone oxime. The authors postulated the latter metabolite to be an artifact formed from the glucuronide of TV-hydroxy cyclohexylamine during the hydrolysis procedure.
In contrast to rabbits, man, as well as rats and guinea pigs, excrete 90% or more of a dose of [14C]-labelled cyclohexylamine unchanged in the urine (Renwick and Williams 1972). Small amounts of radioactivity were found in the feces, 1% or less in man, rat and rabbit, and 4-7% in the guinea pig. Only 4-5% of the dose was metabolized in 24 h in the rat and guinea pig and 1-2% in man. The metabolites identified indicated that in rats, the metabolism of cyclohexylamine was mainly through hydroxylation of the cyclohexane ring, in man by deamination and in guinea pigs and rabbits by ring hydroxylation and deamination. The metabolites to cyclohexylamine were excreted in both free and conjugated forms.
storage
Cyclohexylamine can be stored and shipped in iron tanks. Nonferrous metals, particularly copper-containing materials, are attacked and are therefore unsuitable. The amine discolors on contact with air and therefore must be kept under nitrogen.
Shipping
UN2357 Cyclohexylamine, Hazard class: 8; Labels: 8-Corrosive material, 3-Flammable liquid.
Purification Methods
Dry the amine with CaCl2 or LiAlH4, then distil it from BaO, KOH or Na, under N2. Also purify it by conversion to the hydrochloride (which is crystallised several times from water), then liberation of the amine with alkali and fractional distillation under N2. The hydrochloride has m 205-207o (dioxane/EtOH). [Lycan et al. Org Synth Coll Vol II 319 1943, Beilstein 12 III 10, 12 IV 8.]
Incompatibilities
May form explosive mixture with air. Cyclohexylamine is a strong base: it reacts violently with acid. Contact with strong oxidizers may cause fire and explosion hazard. Incompatible with organic anhydrides; isocyanates, vinyl acetate; acrylates, substituted allyls; alkylene oxides; epichlorohydrin, ketones, aldehydes, alco- hols, glycols, phenols, cresols, caprolactum solution; lead. Corrosive to copper alloys, zinc, or galvanized steel.
Waste Disposal
Incineration; incinerator equipped with a scrubber or thermal unit to reduce nitrogen oxides emissions.
Cyclohexylamine Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34571 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 8672 | 58 |
| Xiamen Eagle Chemical Limited Corporation | +86-5925023701 +86-18900207489 | yiyunchem@163.com | China | 6043 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12446 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5882 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29798 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21667 | 55 |
Related articles
- What are the adverse effects of Cyclohexylamine on humans?
- Cycloheximide is pharmacologically active and acts similarly to reserpine. A single dose administered orally or intravenously ....
- Dec 1,2023
- Application of Cyclohexylamine
- Cyclohexylamine appears as a clear colorless to yellow liquid with an odor of ammonia. Flash point 90°F. Irritates the eyes an....
- Jun 27,2022
View Lastest Price from Cyclohexylamine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-23 | Cyclohexylamine
108-91-8
|
US $10.50 / KG | 1KG | 99% | 10 ton | Hebei Chuanghai Biotechnology Co,.LTD | |
 |
2024-08-29 | Cyclohexylamine
108-91-8
|
US $90.00-85.00 / kilograms | 10kilograms | 99% | 100tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-08-15 | Cyclohexylamine
108-91-8
|
US $30.00-10.00 / kg | 1kg | 99% | 20 tons | Hebei Yanxi Chemical Co., Ltd. |
-

- Cyclohexylamine
108-91-8
- US $10.50 / KG
- 99%
- Hebei Chuanghai Biotechnology Co,.LTD
-

- Cyclohexylamine
108-91-8
- US $90.00-85.00 / kilograms
- 99%
- Hebei Dangtong Import and export Co LTD
-

- Cyclohexylamine
108-91-8
- US $30.00-10.00 / kg
- 99%
- Hebei Yanxi Chemical Co., Ltd.
108-91-8(Cyclohexylamine)Related Search:
1of4