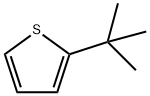
2-T-BUTYLTHIOPHENE synthesis
- Product Name:2-T-BUTYLTHIOPHENE
- CAS Number:1689-78-7
- Molecular formula:C8H12S
- Molecular Weight:140.25
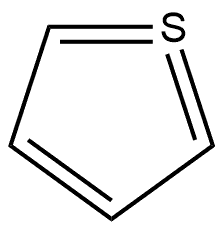
188290-36-0
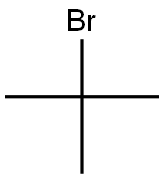
507-19-7

1689-78-7
A dichloromethane (30 mL) solution of thiophene (12.0 g, 0.143 mol) and bromo-tert-butane (19.6 g, 0.143 mol) was slowly added dropwise to a suspension of dichloromethane (30 mL) containing aluminum trichloride (19.1 g, 0.143 mol) at -78 °C for a controlled period of 1 hour. The reaction mixture was stirred continuously at -78 °C for 2 hours, followed by slow warming to room temperature and continued stirring for 18 hours. Upon completion of the reaction, the reaction mixture was diluted with dichloromethane and washed sequentially with water, 5% sodium hydroxide solution and water. The organic layer was dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure to remove the solvent to give the crude product. The crude product was purified by vacuum distillation (20 mmHg) to give 10.7 g of 2-(tert-butyl)thiophene in 53% yield.
Yield: 53%
Reaction Conditions:
with aluminum (III) chloride in dichloromethane at -78 - 20; for 21 h;
Steps:
XIX.1
To a suspension of aluminum trichloride (19.1 g, 0.143 mol) in CH2Cl2 (30 mL) at -78° C. was added, dropwise over 1 h, a solution of thiophene (12.0 g, 0.143 mol) and t-butyl bromide (19.6 g, 0.143 mol) in CH2Cl2 (30 mL). The reaction mixture was stirred at -78° C. for 2 h, then allowed to warm to rt, and stirred for a further 18 h. The reaction mixture was diluted with CH2Cl2, and washed with water, 5% NaOH, and water. The organic layer was dried over Na2SO4 and filtered. Removal of the solvent under reduced pressure afforded a liquid that was purified by vacuum distillation (20 mmHg) to give 10.7 g (53%) of Compound 86
References:
Schering Corporation US2004/10013, 2004, A1 Location in patent:Page/Page column 65

188290-36-0
2 suppliers
inquiry
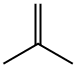
115-11-7
243 suppliers
$45.00/25 g
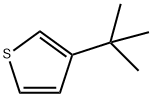
1689-79-8
6 suppliers
inquiry

1689-78-7
49 suppliers
inquiry
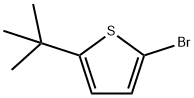
93425-02-6
13 suppliers
inquiry

1689-78-7
49 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)