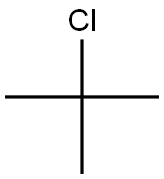
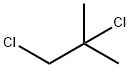
2-Chloro-2-methylpropane synthesis
- Product Name:2-Chloro-2-methylpropane
- CAS Number:507-20-0
- Molecular formula:C4H9Cl
- Molecular Weight:92.57
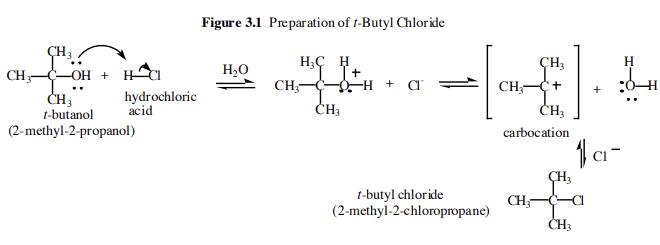
The first step of the overall reaction is an acid-base reaction between the t-butanol and the hydrochloric acid. The t-butanol is a weak base and the hydrochloric acid is a strong acid. The alcoholic oxygen becomes fully protonated and so the equilibrium lies far to the right. In the second step we have the slow loss of water to form a carbocation intermediate. This species is very reactive and is immediately attacked by the chloride ion liberated in the first step to form tert-Butyl chloride.

75-65-0
783 suppliers
$10.00/10ml

507-20-0
384 suppliers
$13.00/25mL
Yield:507-20-0 96.2%
Reaction Conditions:
with acetic acid;calcium chloride at 55; for 8 h;Reagent/catalyst;Temperature;
Steps:
3
200 g of tert-butanol was added to 1000 ml reaction flask, stirred, and then 350 g of glacial acetic acid was added. 200 g of calcium chloride was added, placed in water bath at a temperature of 55°C. The reaction was stirred for 8 hours, allowed to stand, the aqueous layer was separated, and the organic layer obtained by the first stratification was neutralized with 10 g sodium hydroxide, allowed to stand for a second stratification and the aqueous layer was separated. The organic layer obtained by the second layering was dried by adding 40 g of calcium chloride, dried for 1 h, filtered and distilled to obtain 240.3 g tert-butyl chloride. Yield 96.2%. GC 99.8%.
References:
Jinan Chenghui Shuangda Chemical Co., Ltd.;Liu, Yufeng;Hu, JunFeng;Li, Chunjie;Liu, Kai;Tang, Duo CN105399595, 2016, A Location in patent:Paragraph 0018
115-11-7
243 suppliers
$45.00/25 g
594-37-6
66 suppliers
$45.00/1 g

507-20-0
384 suppliers
$13.00/25mL
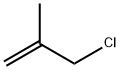
563-47-3
265 suppliers
$10.00/5g

115-11-7
243 suppliers
$45.00/25 g

507-20-0
384 suppliers
$13.00/25mL

563-47-3
265 suppliers
$10.00/5g

115-11-7
243 suppliers
$45.00/25 g

507-20-0
384 suppliers
$13.00/25mL
201230-82-2
1 suppliers
inquiry

75-65-0
783 suppliers
$10.00/10ml
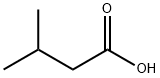
503-74-2
444 suppliers
$5.00/10g

507-20-0
384 suppliers
$13.00/25mL
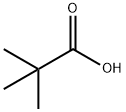
75-98-9
437 suppliers
$10.00/10g