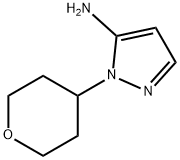
2-(Tetrahydro-pyran-4-yl)-2H-pyrazol-3-ylamine synthesis
- Product Name:2-(Tetrahydro-pyran-4-yl)-2H-pyrazol-3-ylamine
- CAS Number:1157012-67-3
- Molecular formula:C8H13N3O
- Molecular Weight:167.21
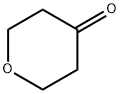
29943-42-8
430 suppliers
$5.00/1g
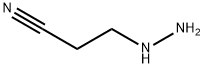
353-07-1
96 suppliers
$24.00/1g

1157012-67-3
36 suppliers
$94.00/100mg
Yield:1157012-67-3 5.34 g
Reaction Conditions:
in ethanol at 20; for 3 h;
Steps:
211.1 Example 211: 4-chioro-1-(tetrahydropyran-4-yl)-1H-pyrazolo[3,4-b]pyridine-5-carboxylic acid (4-chlorophenyl)amide
To a solution of tetrahydropyran-4-one (4.00 g, 40 mmol) in EtOH (40 mL) was added 3-hydrazinopropionitrile (3.40 g, 40 mmol) dropwise at room temperature. The resulting mixture was stirred for 3 hrs at room temperature. Then the mixture was concentrated under reduced pressure and the residue was dissolved in n-butanol (80 mL), followed by the addition of MeONa (4.32 g, 80 mmol). The mixture was refluxed overnight. The mixture was concentrated under reduced pressure. The residue was treated with sat. NH4C1 (80 mL). The aqueous phase was extracted with DCM (60 mL x2) and the extracts were dried over Na2SO4. The solution was concentrated and the residue was purified by Combi flash (CH3CN/H20 = 30%) to give 5.34 g of crude 2-(tetrahydropyran-4-yl)-2H-pyrazol-3-ylamine as a yellow solid. MS: m/z 168.3 (M+H).
References:
WO2016/123392,2016,A2 Location in patent:Paragraph 00544

29943-42-8
430 suppliers
$5.00/1g

1157012-67-3
36 suppliers
$94.00/100mg