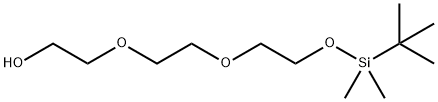
2,2,3,3-tetramethyl-4,7,10-trioxa-3-siladodecan-12-ol synthesis
- Product Name:2,2,3,3-tetramethyl-4,7,10-trioxa-3-siladodecan-12-ol
- CAS Number:201037-95-8
- Molecular formula:C12H28O4Si
- Molecular Weight:264.43

18162-48-6
677 suppliers
$9.00/5g
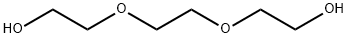
112-27-6
782 suppliers
$6.00/100g

201037-95-8
32 suppliers
$30.00/100mg
Yield: 100%
Reaction Conditions:
Stage #1:2,2'-[1,2-ethanediylbis(oxy)]bisethanol with sodium hydride in tetrahydrofuran;mineral oil at 0 - 20; for 0.666667 h;
Stage #2:tert-butyldimethylsilyl chloride in tetrahydrofuran;mineral oil at 20; for 23 h;
Steps:
64 Production of triethylene glycol mono-tert-butyldimethylsilyl ether
Triethylene glycol (2.2 mL, 16.5 mmol) was added to a solution of 60% sodium hydride (713 mg, 17.8 mmol)in tetrahydrofuran (88 mL) at 0°C and stilled at room temperature for 40 minutes. To this solution, tert-butyldimethylchlorosilane(2.74g, 18.2 mmol) was added, stirred at room temperature for 23 hours, then water was added, andextraction was carried out with dichloromethane. The organic layer was dried with anhydrous sodium sulfate, then thesolvent was distilled away under a reduced pressure to obtain the title compound (4.19 g, quant.) as a pale yellow oilymatter.1H NMR (400 MHz, CDCl3) δ 3.79-3.72 (4H, m), 3.67-3.54 (8H, m), 0.90 (9H, s), 0.07 (6H, s).
References:
Senju Pharmaceutical Co., Ltd.;TAKEDA, Norihiko;MIYABE, Tomoyo;MACHIDA, Shinnosuke;MACHIDA, Mamiko;NAKAJIMA, Takeshi EP3127900, 2017, A1 Location in patent:Paragraph 0256; 0257