tert-butyl 3,8-diazabicyclo[3.2.1]octane-3-carboxylate synthesis
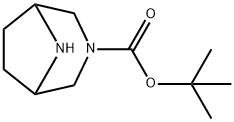
- Product Name:tert-butyl 3,8-diazabicyclo[3.2.1]octane-3-carboxylate
- CAS Number:201162-53-0
- Molecular formula:C11H20N2O2
- Molecular Weight:212.29
![tert-butyl 8-benzyl-3,8-diaza-bicyclo[3.2.1]octane-3-carboxylate](/CAS/GIF/201162-52-9.gif)
201162-52-9
![tert-butyl 3,8-diazabicyclo[3.2.1]octane-3-carboxylate](/CAS/GIF/201162-53-0.gif)
201162-53-0
The general procedure for the synthesis of tert-butyl 3,8-diazabicyclo[3.2.1]octane-3-carboxylate from tert-butyl 8-benzyl-3,8-diazabicyclo[3.2.1]octane-3-carboxylate was as follows: the product of Example 23A (0.62 g, 0.12 mmol) was dissolved in ethanol (10 mL) and palladium/carbon (Aldrich, 60 mg, 10 wt%) as a catalyst. The reaction was stirred at room temperature for 18 h under an atmosphere of hydrogen (balloon) at 1 atm. Upon completion of the reaction, the reaction mixture was filtered to remove the catalyst and the filtrate was concentrated under reduced pressure to remove the solvent. The resulting crude product was purified by silica gel column chromatography with an eluent ratio of 1% ammonia:9% methanol:90% dichloromethane to afford tert-butyl 3,8-diazabicyclo[3.2.1]octane-3-carboxylate, the target compound (0.44 g, 100% yield). Mass spectral analysis (DC1/NH3) showed m/z 213 (M + H)+.
![tert-butyl 8-benzyl-3,8-diaza-bicyclo[3.2.1]octane-3-carboxylate](/CAS/GIF/201162-52-9.gif)
201162-52-9
18 suppliers
inquiry
![tert-butyl 3,8-diazabicyclo[3.2.1]octane-3-carboxylate](/CAS/GIF/201162-53-0.gif)
201162-53-0
136 suppliers
$18.00/100mg
Yield:201162-53-0 100%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in ethanol; under 760.051 Torr; for 18 h;
Steps:
23.B 3,8-Diaza-bicyclo[3.2.1]octane-3-carboxylic Acid tert-butyl Ester
Example 23B 3,8-Diaza-bicyclo[3.2.1]octane-3-carboxylic Acid tert-butyl Ester A solution of the product of Example 23A (0.62 g. 0.12 mmol) in EtOH (10 mL) was stirred with Pd/C (Aldrich, 60 mg, 10 wt %) under 1 atmosphere of H2 (balloon) for 18 h. The mixture was filtered, concentrated and purified by column chromatography (SiO2, 1% NH4OH: 9% CH3OH: 90% CH2Cl2) to provide the title compound (0.44 g, 100% yield). MS (DCl/NH3) m/z 213 (M+H)+.
References:
US2005/101602,2005,A1 Location in patent:Page/Page column 35
![8-Benzyl-3,8-diaza-bicyclo[3.2.1]octane](/CAS/GIF/93428-56-9.gif)
93428-56-9
54 suppliers
$70.00/10mg
![tert-butyl 3,8-diazabicyclo[3.2.1]octane-3-carboxylate](/CAS/GIF/201162-53-0.gif)
201162-53-0
136 suppliers
$18.00/100mg
![1-Propanone, 1-[8-(phenylmethyl)-3,8-diazabicyclo[3.2.1]oct-3-yl]-](/CAS/20210305/GIF/98068-06-5.gif)
98068-06-5
0 suppliers
inquiry
![tert-butyl 3,8-diazabicyclo[3.2.1]octane-3-carboxylate](/CAS/GIF/201162-53-0.gif)
201162-53-0
136 suppliers
$18.00/100mg
![3,8-Diazabicyclo[3.2.1]octane, 3-(1-oxopropyl)-](/CAS/20180531/GIF/67571-93-1.gif)
67571-93-1
0 suppliers
inquiry
![tert-butyl 3,8-diazabicyclo[3.2.1]octane-3-carboxylate](/CAS/GIF/201162-53-0.gif)
201162-53-0
136 suppliers
$18.00/100mg