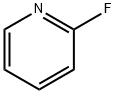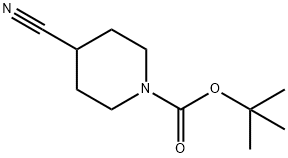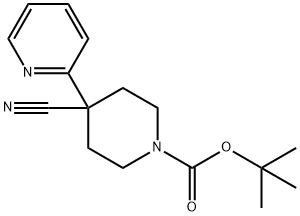
TERT-BUTYL 4-CYANO-4-(PYRIDIN-2-YL)PIPERIDINE-1-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 4-CYANO-4-(PYRIDIN-2-YL)PIPERIDINE-1-CARBOXYLATE
- CAS Number:167263-04-9
- Molecular formula:C16H21N3O2
- Molecular Weight:287.36
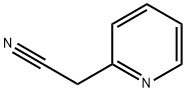
2739-97-1
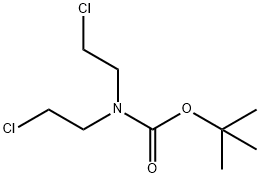
118753-70-1

167263-04-9
Sodium hydride (60% dispersed in mineral oil, 2.04 g, 51 mmol) was added in batches to a stirred solution of 2-pyridineacetonitrile (1.8 mL, 17 mmol) and tert-butyl N,N-bis(2-chloroethyl)carbamate (in appropriate amounts) in anhydrous DMF (50 mL) under nitrogen protection, and the temperature of the reaction was maintained at 0°C. The reaction was carried out in a controlled manner. The addition process was controlled to be completed within 8 min. Subsequently, the reaction system was warmed up to 60 °C and the reaction was continuously stirred for 5.5 hours. After completion of the reaction, the mixture was cooled to room temperature, extracted with ethyl acetate (4 x 150 mL), the organic phases were combined and washed with water (3 x 200 mL). The organic layer was dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated under reduced pressure to give a red to black oily crude product. The crude product was adsorbed on silica gel and purified by column chromatography with the eluent being an isohexane solution of 20% ethyl acetate to give tert-butyl 4-cyano-4-(pyridin-2-yl)piperidine-1-carboxylate as an orange solid (2.13 g, 44% yield). The structure of the product was confirmed by 1H NMR (360 MHz, CDCl3): δ 1.48 (9H, s), 2.04-2.08 (2H, m), 2.17-2.25 (2H, m), 3.15-3.28 (2H, m), 4.20-4.35 (2H, m), 7.25-7.29 (1H, m), 7.61 (1H, d, J = 8 Hz), 7.73-7.78 (1H, m), 8.61 (1H, dd, J = 0.7, 3.9 Hz). The mass spectrum (MS) showed [M+H]+ m/z = 287 (corresponding to the loss of the tert-butyl fragment, -56).
Yield:167263-04-9 89%
Reaction Conditions:
with lithium hexamethyldisilazane in tetrahydrofuran at -78 - 20;Inert atmosphere;
References:
Chang, Ronald K.;Di Marco, Christina N.;Pitts, Daniel R.;Greshock, Thomas J.;Kuduk, Scott D. [Tetrahedron Letters,2009,vol. 50,# 46,p. 6303 - 6306] Location in patent:experimental part

2739-97-1
212 suppliers
$10.00/1g

118753-70-1
178 suppliers
$6.00/1g

167263-04-9
34 suppliers
inquiry

118753-70-1
178 suppliers
$6.00/1g

167263-04-9
34 suppliers
inquiry