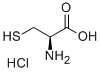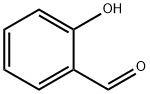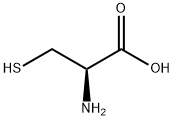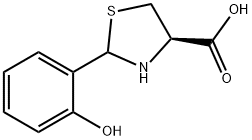
(R)-2-(2-Hydroxyphenyl)thiazolidine-4-carboxylic acid synthesis
- Product Name:(R)-2-(2-Hydroxyphenyl)thiazolidine-4-carboxylic acid
- CAS Number:201942-90-7
- Molecular formula:C10H11NO3S
- Molecular Weight:225.26
Yield:201942-90-7 95%
Reaction Conditions:
with sodium hydrogencarbonate in ethanol;water; for 6 h;
Steps:
5.1.1. (2RS,4R)-2-Phenyl-thiazolidine-4-carboxylic acid (1a)
General procedure: To the solution of l-cysteine hydrochloride hydrate and NaHCO3 (1.1 equiv) in water (200 mL), benzaldehyde (1.1 equiv) in 95% ethanol (200 mL) was added in one portion. The reaction mixture was stirred for 6 h. The product was filtered, washed with ethanol, and dried to afford as a white solid.
References:
Liu, Yu;Jing, Fanbo;Xu, Yingying;Xie, Yuanchao;Shi, Fangyuan;Fang, Hao;Li, Minyong;Xu, Wenfang [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 7,p. 2342 - 2348] Location in patent:experimental part