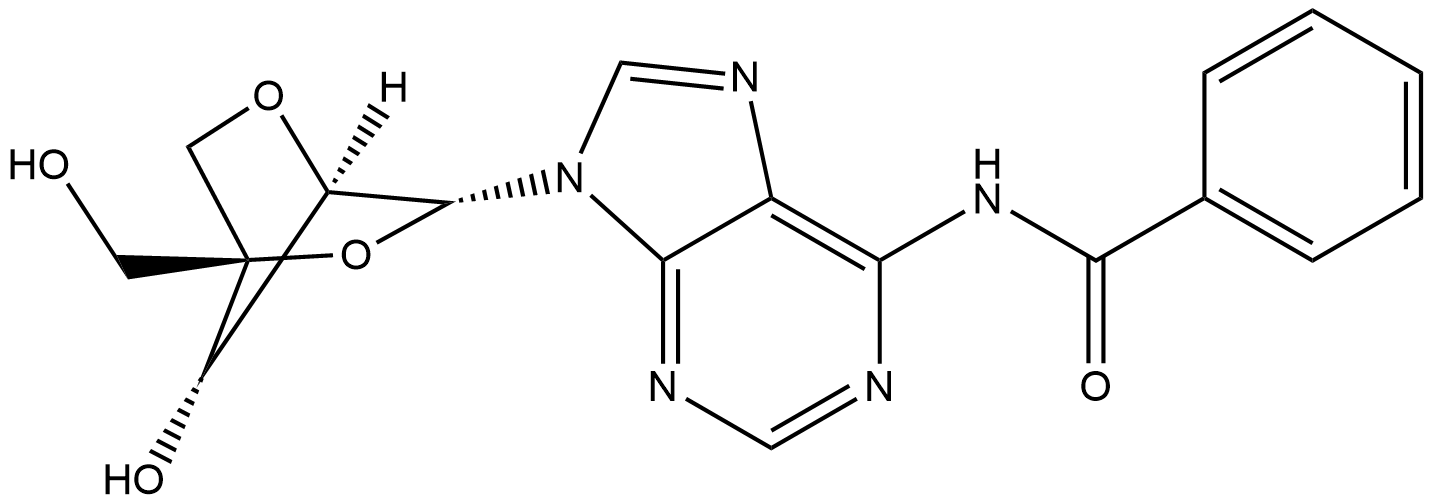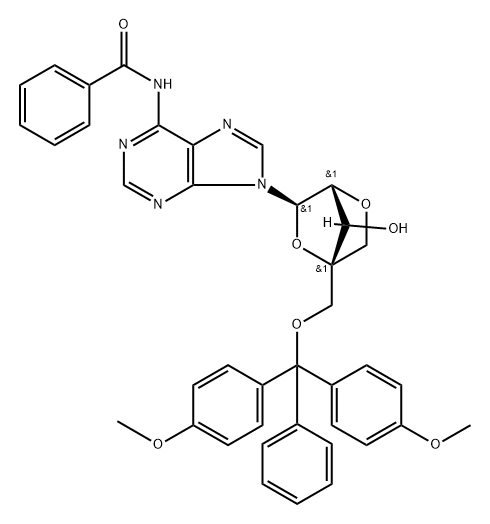
Benzamide, N-[9-[2,5-anhydro-4-C-[[bis(4-methoxyphenyl)phenylmethoxy]methyl]-α-L-lyxofuranosyl]-9H-purin-6-yl]- synthesis
- Product Name:Benzamide, N-[9-[2,5-anhydro-4-C-[[bis(4-methoxyphenyl)phenylmethoxy]methyl]-α-L-lyxofuranosyl]-9H-purin-6-yl]-
- CAS Number:206055-74-5
- Molecular formula:C39H35N5O7
- Molecular Weight:685.74
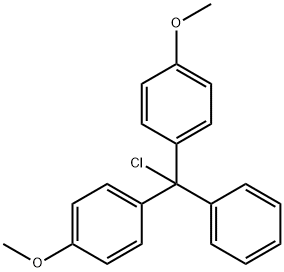
40615-36-9
547 suppliers
$5.00/1g
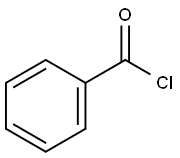
98-88-4
598 suppliers
$10.00/5g
![9-[2,5-Anhydro-4-C-(hydroxymethyl)-alpha-L-lyxofuranosyl]-9H-purin-6-amine](/CAS/20180808/GIF/206055-70-1.gif)
206055-70-1
41 suppliers
inquiry
![Benzamide, N-[9-[2,5-anhydro-4-C-[[bis(4-methoxyphenyl)phenylmethoxy]methyl]-α-L-lyxofuranosyl]-9H-purin-6-yl]-](/CAS/20211123/GIF/206055-74-5.gif)
206055-74-5
20 suppliers
inquiry
Yield:206055-74-5 95%
Reaction Conditions:
Stage #1: 4,4'-dimethoxytrityl chloride;(1S,3R,4R,7S)-3-(6-amino-9H-purin-9-yl)-1-(hydroxymethyl)-2,5-dioxabicyclo[2.2.1]heptan-7-olwith pyridine at 20; for 3 h;Inert atmosphere;Large scale;
Stage #2: with chloro-trimethyl-silane at 0; for 1 h;Large scale;
Stage #3: benzoyl chlorideLarge scale;Further stages;
Steps:
3.3.13 3.13 Synthesis of b--A-7
3.13.1 Process description P-D-A-6 (1000 g) is dissolved in anh. pyridine (30 L) under nitrogen atmosphere and DMTrCl (2014 g) is added. The deep red reaction mixture is stirred at room temperature until TLC analysis (10 % MeOH in DCM) shows full conversion (typical 3 h). Reaction cooled to 0°C and TMSC1 (2064 g) is added over a period of 1 h. Benzoylchloride (2670 g) is added at 0°C, and the reaction mixture is allowed to warm up to 20 - 25°C and left overnight. The beige suspension is cooled to 0°C and MeOH (5.0 L) is added drop wise at max. l0°C (exothermic, jacket set to minus l0°C). A mixture of 25% NH3 aq (8300 mL) and purified water (3.0 L) is added to the mixture (exothermic) at max. 20°C. The reaction mixture is stirred at room temperature until TLC analysis (10 % MeOH in DCM) shows full conversion to TM (typical 5 h). The reaction is added water (5.0 L) and toluene (20.0 L). The phases are separated and the organic phase is concentrated under reduced pressure using a rotary evaporator (water bath 60°C). 3.13.2 Purification The purification was performed by chromatography: The crude oil is dissolved in 1 :1 EtOAc/ Toluene + 0.1% TEA. Silica gel is washed with 1 : 1 EtOAc/ Toluene + 0.5% TEA. Eluent: 1 : 1 EtOAc/ Toluene + 0.1% TEA until impurities are out 7:1 EtOAc/ THF + 0.1% TEA to eluate b--A-7 out. The product containing fractions are pooled and concentrated under reduced pressure to give white crispy crystals. Typical yield: 90 - 95 % white crystals (2300 g) 3.13.3 Analytical Methods: TLC Analysis: Merck Silica gel 60, F254 , on aluminium foil. Eluent: 10 % MeOH in DCM DetectiomUV and spraying with H2SO4/ MeOH (1 :1) + charring. HPLC: ColummXTerra, MS C-18, 5 pm, 2.1 x 100 mm Temp.:40°C Flow: 0.3 mL/ min DetectiomUV at 254 nm Inj. Vol.:3 pL Sample prep.: 0.1 mg/ mL in CH3CN Integ. time: 14 min Eluent: A: 0.1 % sat. NH4OH (aq) in H2O B: 20 % A in CHsCN Gradient: b--A-7 Rt : 1.75 min MS - ESI: [M+H]+ found: 686.2 Theor. mass: 686.2 3.13.4 Optional steps 1) If any silylated intermediates is observed in the organic phase, an aqueous potassium fluoride solution is added at 40°C and the mixture is stirred until all silylated intermediates is deprotected. 2) If any di-benzoylated intermediates is observed in the organic phase a 25% aqueous ammonia solution is added and the mixture is stirred at 25°C. 3) In both cases the organic phase is washed twice with water
References:
WO2019/224172,2019,A1 Location in patent:Page/Page column 42-43; 67-70
63593-02-2
5 suppliers
inquiry
![Benzamide, N-[9-[2,5-anhydro-4-C-[[bis(4-methoxyphenyl)phenylmethoxy]methyl]-α-L-lyxofuranosyl]-9H-purin-6-yl]-](/CAS/20211123/GIF/206055-74-5.gif)
206055-74-5
20 suppliers
inquiry
63593-03-3
161 suppliers
$38.00/250mg
![Benzamide, N-[9-[2,5-anhydro-4-C-[[bis(4-methoxyphenyl)phenylmethoxy]methyl]-α-L-lyxofuranosyl]-9H-purin-6-yl]-](/CAS/20211123/GIF/206055-74-5.gif)
206055-74-5
20 suppliers
inquiry