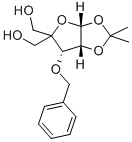
3-O-Benzyl-4-(hydroxymethyl-1,2-O-isopropylidene)-alpha-D-erythropentofuranose synthesis
- Product Name:3-O-Benzyl-4-(hydroxymethyl-1,2-O-isopropylidene)-alpha-D-erythropentofuranose
- CAS Number:63593-03-3
- Molecular formula:C16H22O6
- Molecular Weight:310.34

50-00-0
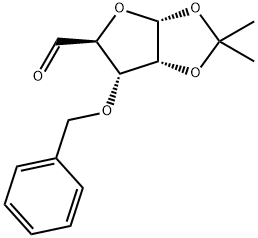
63593-02-2

63593-03-3
The general procedure for the synthesis of ((3aR,6S,6aR)-6-(benzyloxy)-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxazole-5,5-diyl)dimethanol from formaldehyde and the compound (CAS: 63593-02-2) was as follows: to a 3000 mL round-bottomed flask at room temperature was added compound 269-4 (100 g 359.32 mmol, 1.00 equiv) to a solution of tetrahydrofuran (500 mL). Water (500 mL) was then added. After the mixture was cooled to 0 °C, sodium hydroxide solution (600 mL, 2N aqueous solution) was slowly added, followed by aqueous formaldehyde solution (240 mL, 37%). The reaction mixture was stirred at room temperature overnight. After the reaction was completed, the mixture was diluted with ethyl acetate (1500 mL) and washed with saturated aqueous sodium bicarbonate solution. Finally, the mixture was washed with aqueous sodium chloride solution (3 x 500 mL).

50-00-0
896 suppliers
$10.00/25g

63593-02-2
5 suppliers
inquiry

63593-03-3
161 suppliers
$38.00/250mg
Yield:63593-03-3 75%
Reaction Conditions:
with sodium hydroxide in 1,4-dioxane at 28 - 30; for 16.83 h;
Steps:
3.3.5 3.5.3 Process description step 2
Dioxane (2.0 L) is added to the residue and the resulting turbid solution is filtered on a GF-P3, and the filter cake is washed with dioxane (0.3 L). The yellow clear filtrate is transferred into a reactor together with 37 % formaldehyde (700 mL). 2 N Sodium hydroxide (2.3 L) is added over a period of 50 min. The temperature is kept below 30°C by occasional cooling of the mixture. The mixture is stirred at 28°C until TLC (eluent: 1/1 EtOAc/ heptane, detected by H2SO4 + heat) shows full conversion to P-D-S-6 (typical 16 h). 3.5.4 Purification step 2 Sodium chloride (0.3 kg) is added and the mixture is stirred until the NaCl has dissolved. The phases are separated, and the aqueous phase is extracted with DCM (2 x 1.5 L). The combined organic phases are washed with 20 % sodium chloride (1.5 L). Additional sodium chloride (150 g) is added to improve phase separation. The combined organic phases are concentrated using a rotary evaporator, finally at 60°C and 30 mbar. Warm MTBE (3 L, app 50°C) is added to the residue and the hot solution (45-50°C) is transferred to a glass reactor. Heptane (1.5 L) is added and the mixture allowed to cool down to room temperature. The first crop of crystals are isolated on a GF-P3, and the filter cake is washed with MTBE/ heptane (1 :3) (1.5 L, 20°C). The filtrate is evaporated, and MTBE (500 mL) and heptane (200 mL) is added to the residue. The hot solution (45-50°C) is transferred to a flask and allowed to reach RT. The secound crop of crystals are isolated on a GF-P3. The material is left to dry in a vacuum oven over night to give 750 g (yield: 75 %) white solid. 3.5.5 Analytical method TLC (eluent: EtO Ac/heptane 1 :1): P-D-S-5: Rf= 0.20 Aldehyde intermediate (smear): Rf= 0.40 P-D-S-6: Rf= 0.20
References:
WO2019/224172,2019,A1 Location in patent:Page/Page column 39; 50-52
![(3aR,6aR)-5-[(4R)-2,2-dimethyl-1,3-dioxolan-4-yl]-2,2-dimethyl-3a,5,6,6a-tetrahydrofuro[2,3-d][1,3]dioxol-6-ol:methane](/CAS/20180529/GIF/620630-82-2.gif)
620630-82-2
3 suppliers
inquiry

63593-03-3
161 suppliers
$38.00/250mg
57099-04-4
21 suppliers
inquiry

63593-03-3
161 suppliers
$38.00/250mg
22331-21-1
87 suppliers
$70.00/10g

63593-03-3
161 suppliers
$38.00/250mg

100-39-0
429 suppliers
$10.00/10g

63593-03-3
161 suppliers
$38.00/250mg