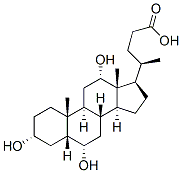
(3a,5b,6a,12a)-3,6,12-trihydroxy-Cholan-24-oic acid synthesis
- Product Name:(3a,5b,6a,12a)-3,6,12-trihydroxy-Cholan-24-oic acid
- CAS Number:21066-18-2
- Molecular formula:C24H40O5
- Molecular Weight:408.57
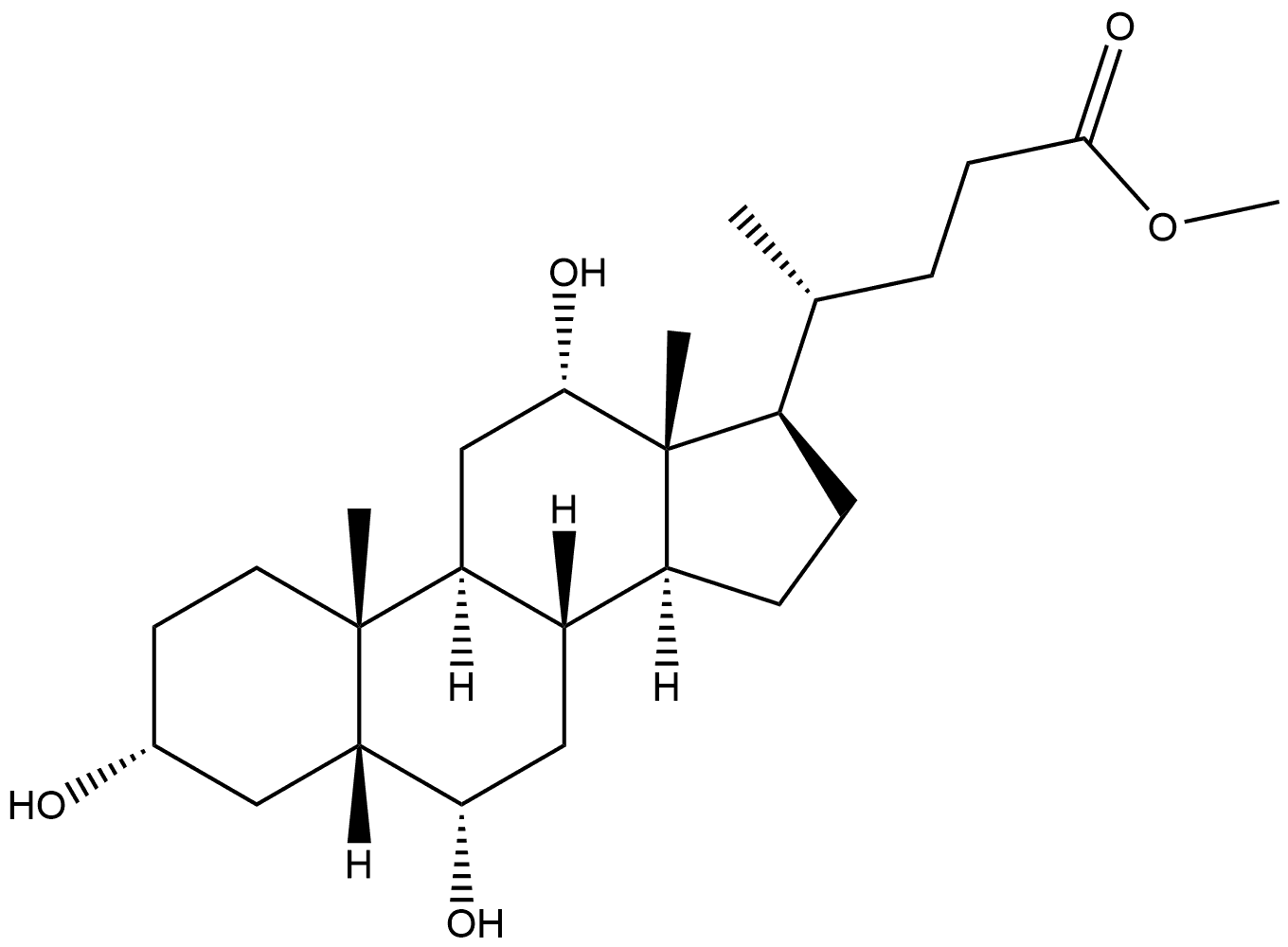
134526-11-7
0 suppliers
inquiry

21066-18-2
1 suppliers
inquiry
Yield:21066-18-2 70%
Reaction Conditions:
with lithium hydroxide monohydrate;water in tetrahydrofuran;methanol at 20;
Steps:
The eleventh step: hydrolysis
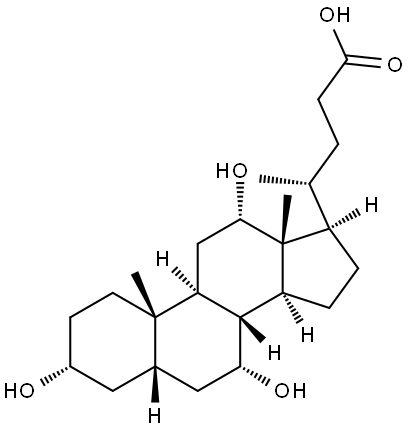
General procedure: 3mg (0.007mmol) 3α, 6α, 12α-trihydroxy-5β-cholic acid methyl ester 18a/18mg (0.04mmol) 3α, 6β, 12α-trihydroxy-5β-cholic acid methyl ester 18b was dissolved in 0.5ml/1.5 In a mixed solvent of ml tetrahydrofuran, 0.3ml/0.9ml methanol, and 0.2ml/0.6ml water, 1mg/4mg lithium hydroxide monohydrate is added, and the reaction is stirred at room temperature until the reaction is detected by thin layer chromatography.Use acid-base extraction method, ethyl acetate extraction, saturated brine washing, anhydrous sodium sulfate drying, and concentration under reduced pressure. The crude product obtained is subjected to flash silica gel column chromatography (dichloromethane: acetone=1:2) to obtain a white solid, namely 3α , 6α,12α-trihydroxy-5β-cholic acid 7a (2mg, 70%)/3α,6β,12α-trihydroxy-5β-cholic acid 7b (12mg, 70%).
References:
CN111718389,2020,A Location in patent:Paragraph 0039
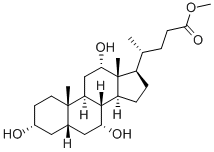
81-25-4
570 suppliers
$5.00/100mg

21066-18-2
1 suppliers
inquiry
1448-36-8
172 suppliers
$5.00/100mg

21066-18-2
1 suppliers
inquiry

108322-63-0
0 suppliers
inquiry

21066-18-2
1 suppliers
inquiry