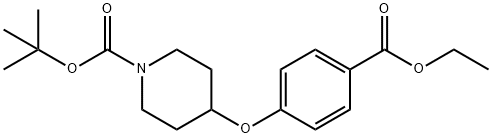
tert-Butyl 4-(4-(ethoxycarbonyl)phenoxy)piperidine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-(4-(ethoxycarbonyl)phenoxy)piperidine-1-carboxylate
- CAS Number:210962-44-0
- Molecular formula:C19H27NO5
- Molecular Weight:349.42
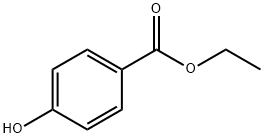
120-47-8
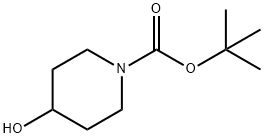
109384-19-2

210962-44-0
Step 1: At 0°C, DEAD (12.3 mL, 27.08 mmol, 40% toluene solution) was slowly added dropwise to THF containing ethyl 4-hydroxybenzoate (3.0 g, 18.05 mmol), N-Boc-4-hydroxypiperidine (4.72 g, 23.47 mmol), and triphenylphosphine (6.16 g, 23.47 mmol) ( 40 mL) solution. The reaction mixture was stirred at 0 °C to room temperature for 24 hours. Subsequently, the reaction solution was concentrated under reduced pressure. The residue was diluted with EtOAc, washed sequentially with 1N NaOH and saturated saline, and the organic phase was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (elution gradient: 0→30% EtOAc/hexane) to afford tert-butyl 4-(4-(ethoxycarbonyl)phenoxy)piperidine-1-carboxylate (5.4 g, 15.45 mmol, 85.6% yield) as a colorless oil.ESIMS m/z: 372.1 [M+Na]+ (calculated value C19H27NO5Na. 372.1).

120-47-8
722 suppliers
$5.00/100mg

109384-19-2
426 suppliers
$5.00/1g

210962-44-0
24 suppliers
inquiry
Yield:210962-44-0 85.6%
Reaction Conditions:
with triphenylphosphine;diethylazodicarboxylate in tetrahydrofuran;toluene at 0 - 20; for 24 h;
Steps:
1 Step 1
Step 1
To a solution of DEAD (12.3 mL, 27.08 mmol)(40% in toluene)was added to a mixture of ethyl 4-hydroxybenzoate (XIII)(3.0 g, 18.05 mmol), tert-butyl 4-hydroxypiperidine-1-carboxylate (XI)(4.72 g, 23.47 mmol)and triphenylphosphane (6.16 g, 23.47 mmol)in THF (40 mL)at 0° C.
The mixture was stirred from 0° C. to room temperature over 1 day before concentrating in vacuo.
The residue was diluted with EtOAc, washed with 1 N NaOH and brine, and then evaporated under vacuum.
The crude product was purified by chromatography (0→30% EtOAc/hexanes)to give tert-butyl 4-(4-ethoxycarbonylphenoxy)piperidine-1-carboxylate (XIV)(5.4 g, 15.45 mmol, 85.6% yield)as a colorless oil. ESIMS found for C19H27NO5 m/z 372.1 (M+Na).
References:
US2017/313682,2017,A1 Location in patent:Paragraph 0702; 0703

120-47-8
722 suppliers
$5.00/100mg

109384-19-2
426 suppliers
$5.00/1g
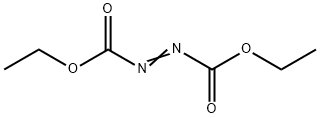
1972-28-7
392 suppliers
$15.00/5g

210962-44-0
24 suppliers
inquiry