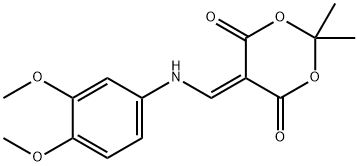
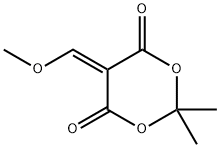
1,3-Dioxane-4,6-dione, 5-[[(3,4-diMethoxyphenyl)aMino]Methylene]-2,2-diMethyl- synthesis
- Product Name:1,3-Dioxane-4,6-dione, 5-[[(3,4-diMethoxyphenyl)aMino]Methylene]-2,2-diMethyl-
- CAS Number:213699-53-7
- Molecular formula:C15H17NO6
- Molecular Weight:307.3
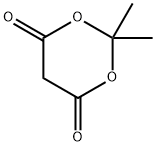
2033-24-1
656 suppliers
$6.00/25g
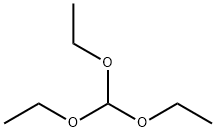
122-51-0
510 suppliers
$10.00/5ml
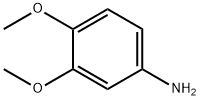
6315-89-5
310 suppliers
$7.00/10g
![1,3-Dioxane-4,6-dione, 5-[[(3,4-diMethoxyphenyl)aMino]Methylene]-2,2-diMethyl-](/CAS/20150408/GIF/213699-53-7.gif)
213699-53-7
18 suppliers
inquiry
Yield:213699-53-7 91.5%
Reaction Conditions:
in ethanol at -20;Reflux;
Steps:
1.1 Example 1 N-('4-('('6J-dimethoxyquinolin-4-yl oxy phenyl -l,5-dimethyl-3-oxo-2 -phenyl -2,3-dihydro-lH-pyrazole-4-carboxamide Step 1) 5-('('('3,4-dimethoxyphenyl amino methylene -2,2-dimethyl-l ,3-dioxane-4,6-dione
[0168] To a solution of 3,4-dimethoxyaniline (50 g, 0.33 mol) and 2,2-dimethyl- I, 3-dioxane-4,6-dione (56.5 g, 0.39 mol) in anhydrous EtOH (100 mL) was added triethoxymethane (53.2 g, 0.36 mol). The reaction was heated to reflux for 1 hour, then cooled down to -20 °C and stirred further for 2 hours. The solid formed was collected through filtration and washed with EtOH (100 mL) to afford the title compound as a gray solid (91.5 g, 91.5%). MS (ESI, neg. ion) m/z: 306.2 [M-H]"; FontWeight="Bold" FontSize="10" H NMR (400 MHz, CDC13): δ 1.76 (s, 6H), 3.90 (s, 3H), 3.93 (s, 3H), 6.76 (d, J= 2.5 Hz, 1H), 6.81 (dd, J= 2.5 Hz, 7.0 Hz, 1H), 6.89 (d, J = 8.2 Hz, 1H), 8.55 (d, J= 14.4 Hz, 1H), I I.24 (d, J= 14.3 Hz, 1H).
References:
WO2013/180949,2013,A1 Location in patent:Paragraph 0168

2033-24-1
656 suppliers
$6.00/25g

6315-89-5
310 suppliers
$7.00/10g
![1,3-Dioxane-4,6-dione, 5-[[(3,4-diMethoxyphenyl)aMino]Methylene]-2,2-diMethyl-](/CAS/20150408/GIF/213699-53-7.gif)
213699-53-7
18 suppliers
inquiry

2033-24-1
656 suppliers
$6.00/25g

6315-89-5
310 suppliers
$7.00/10g
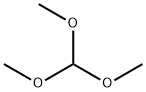
149-73-5
541 suppliers
$12.00/5g
![1,3-Dioxane-4,6-dione, 5-[[(3,4-diMethoxyphenyl)aMino]Methylene]-2,2-diMethyl-](/CAS/20150408/GIF/213699-53-7.gif)
213699-53-7
18 suppliers
inquiry
15568-85-1
161 suppliers
$7.00/1g

6315-89-5
310 suppliers
$7.00/10g
![1,3-Dioxane-4,6-dione, 5-[[(3,4-diMethoxyphenyl)aMino]Methylene]-2,2-diMethyl-](/CAS/20150408/GIF/213699-53-7.gif)
213699-53-7
18 suppliers
inquiry

2033-24-1
656 suppliers
$6.00/25g
![1,3-Dioxane-4,6-dione, 5-[[(3,4-diMethoxyphenyl)aMino]Methylene]-2,2-diMethyl-](/CAS/20150408/GIF/213699-53-7.gif)
213699-53-7
18 suppliers
inquiry