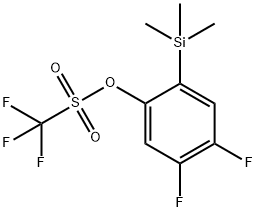
217813-00-8 synthesis
- Product Name:217813-00-8
- CAS Number:217813-00-8
- Molecular formula:C10H11F5O3SSi
- Molecular Weight:334.34
Yield: 50.6%
Reaction Conditions:
Stage #1:(2-bromo-4,5-difluorophenyl) trimethylsilane with n-butyllithium in tetrahydrofuran;hexane at -78; for 0.333333 h;
Stage #2:trifluoromethylsulfonic anhydride in tetrahydrofuran;hexane at -78; for 0.333333 h;
Steps:
1.2 4,5-Difluoro-2-(trimethylsilyl)phenyl trifluoromethanesulfonate (N3)
To a stirred solution of (2-bromo-4,5-difluorophenyl)trimethylsilane (12.6 g, 47.5 mmol) in THF (30 mL) was added dropwise n-BuLi (19 mL, 2.5 M in hexanes) at -78 ^. The reaction mixture was stirred for 20 min at -78 ^. Tf2O (19.9 g, 95.0 mmol) was added dropwise to the reaction mixture, and kept stirring for 20 min under -78 ^. The reaction was quenched with cold saturated aqueous NaHCO3 and the resulting mixture was extracted with EtOAc (100 mL*3). The combined organic extracts were dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by silica gel (PE: EA=100: 1) to afford N3 (8 g, Y: 50.6%) as colorless oil. 1H NMR (400 MHz, CDCl3): δ 7.32 - 7.21 (m, 2H), 0.36 (s, 9H).1H NMR: BONB-00112-159-1
References:
BLUE OAK PHARMACEUTICALS, INC. WO2021/138314, 2021, A1 Location in patent:Paragraph 0165; 0234-0235
![Benzene, 1-bromo-4,5-difluoro-2-[(trimethylsilyl)oxy]-](/CAS/20210305/GIF/232611-13-1.gif)
232611-13-1
0 suppliers
inquiry

217813-00-8
28 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)