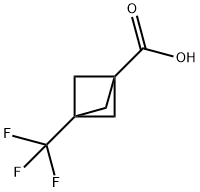
3-(Trifluoromethyl)bicyclo[1.1.1]pentane-1-carboxylicacid synthesis
- Product Name:3-(Trifluoromethyl)bicyclo[1.1.1]pentane-1-carboxylicacid
- CAS Number:224584-18-3
- Molecular formula:C7H7F3O2
- Molecular Weight:180.12

124-38-9
134 suppliers
$175.00/23402
![Bicyclo[1.1.1]pentane, 1-iodo-3-(trifluoromethyl)-](/CAS/20180527/GIF/119934-12-2.gif)
119934-12-2
35 suppliers
inquiry
![3-(Trifluoromethyl)bicyclo[1.1.1]pentane-1-carboxylicacid](/CAS/20180629/GIF/224584-18-3.gif)
224584-18-3
62 suppliers
$338.00/10mg
Yield: 86%
Reaction Conditions:
Stage #1:1-(trifluoromethyl)-3-iodobicyclo<1.1.1>pentane with tert.-butyl lithium;sodium hydrogencarbonate in diethyl ether;pentane at -78; for 0.5 h;
Stage #2:carbon dioxide in diethyl ether;pentane at -78 - 20; for 0.166667 h;
Steps:
2
[0177] l-(Trif].uorom.ethy].)-3-iodobicyclo[l.l.Ijpemtane (1.02 g, 3.89 mmol) was dissolved in anhydrous diethyl ether (13.0 mL) and cooled to -78 °C. A solution of tBuLi (1.7 M in pentane, 0.549 g, 8.56 mmol, 5.04 mL) was added slowly, and the solution was stirred at -78 °C. After 30 mins, CO? was bubbled through the solution for 10 mins, and the reaction was allowed to warm to rt. Diethyl ether (10 mL) was added, and the mixture was extracted with H?Q (3 x 20 mL). The combined aqueous layers were acidified with 2M HCl then extracted with Et?0 (3 x 20 mL). The combined organics were dried (MgS04) and concentrated under reduced pressure to afford 2b (0.603g, 86%) as a white solid which was carried forward without further purification. 1HNMR (400 MHz, DMSO-d6) δ 12.77 (br s,COO//. 1 i n. 2.20 is. 6 H .
References:
KALYRA PHARMACEUTICALS, INC.;BUNKER, Kevin, Duane;GUO, Chuangxing;GRIER, Mark, Charles;HOPKINS, Chad, Daniel;PINCHMAN, Joseph, Robert;SLEE, Deborah, Helen;HUANG, Qinhua;KAHRAMAN, Mehmet WO2016/44331, 2016, A1 Location in patent:Paragraph 0177