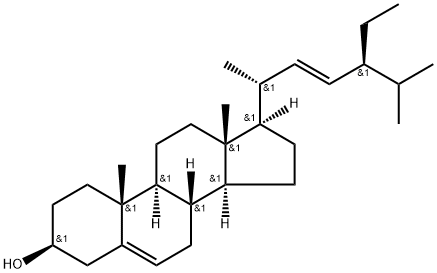
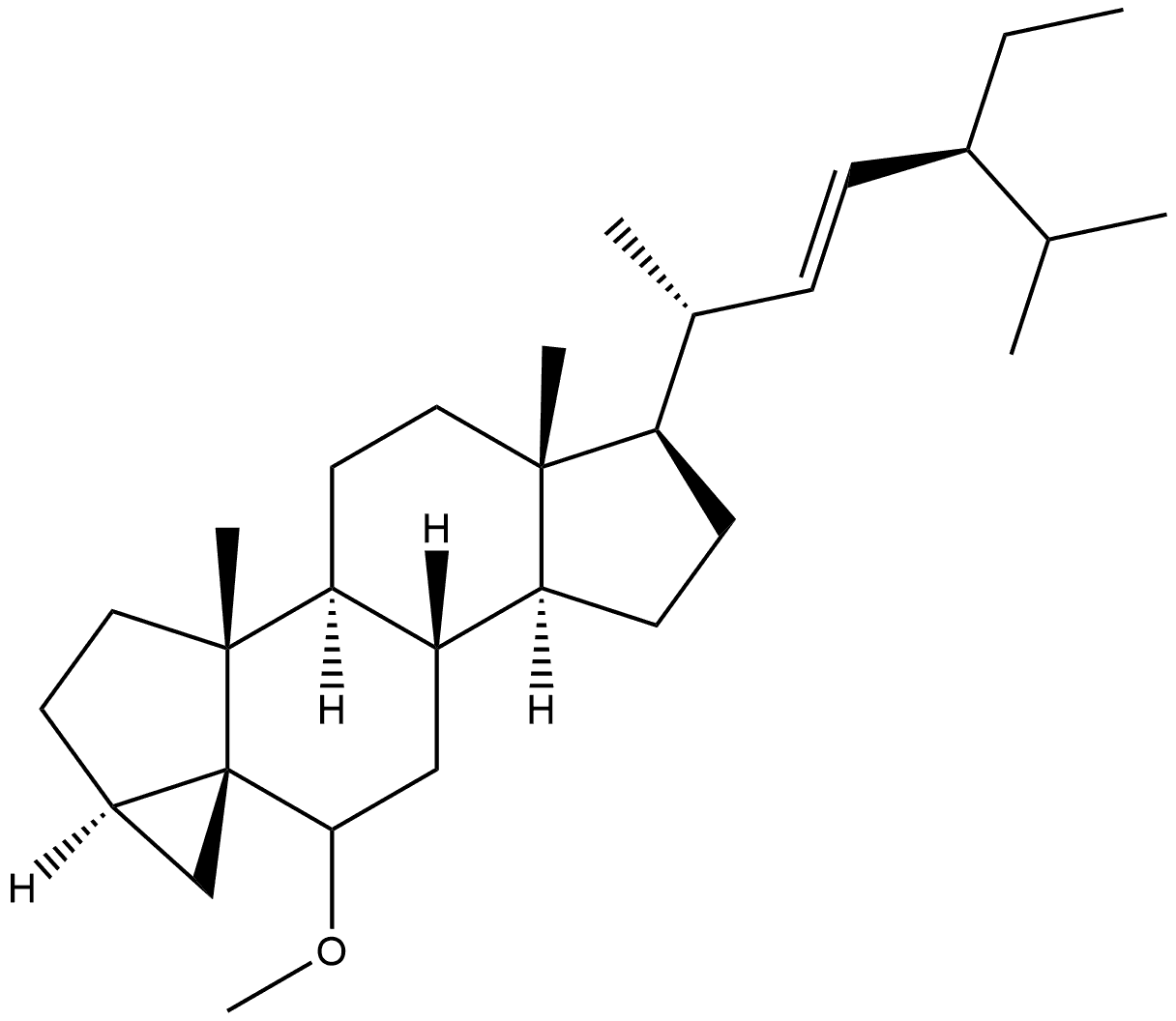
(22E)-3β-Methoxystigmasta-5,22-diene synthesis
- Product Name:(22E)-3β-Methoxystigmasta-5,22-diene
- CAS Number:10453-25-5
- Molecular formula:C30H50O
- Molecular Weight:426.73
Yield:10453-25-5 89.9%
Reaction Conditions:
with potassium acetate for 2 h;Reflux;Inert atmosphere;
Steps:
β-Sitosterol (16)
General procedure: A mixture of 15 (5.0 g, 12.1 mmol) and p-TsCl (6.0 g, 31.5 mmol) in pyridine(70 mL) was stirred at room temperature for 24 h. The mixture was poured into cold saturatedNaHCO3. The resulting solid was collected by filtration, washed with H2O, and dried in a vacuumoven at 60 °C. Recrystallization from acetone gave tosylate (6.267 g, 91.2%) as colorless crystals: mp137-140 °C (lit.22 mp 147-148 °C). []D29 -47.91 (c 1.274, CHCl3) (lit.22 []D -49 (c 1, CHCl3)). 1HNMR (CDCl3): 7.79 (2H, d, J=8.3 Hz), 7.32 (2H, d, J=8.0 Hz), 5.32-5.28 (1H, m), 5.14 (1H, dd,J=15.2, 8.5 Hz), 5.01 (1H, dd, J=15.2, 8.6 Hz), 4.38-4.28 (1H, m), 2.48-2.38 (1H, m), 2.44 (3H, s), 2.27(1H, ddd, J=13.2, 5.1, 1.8 Hz), 2.08-1.90 (3H, m), 1.85-1.78 (2H, m), 1.75-1.64 (2H, m), 1.01 (3H, d,J=6.6 Hz), 0.97 (3H, s), 0.85-0.78 (9H, m), 0.68 (3H, s). A mixture of KOAc (4.50 g, 45.9 mmol) and the above-mentioned tosylate (4.45 g, 7.86 mmol) in dry MeOH (200 mL) was refluxed for 2 h. Afterremoval of the solvent under reduced pressure, the residue was extracted with AcOEt, washed with H2O,saturated aq. NaHCO3, and saturated aq. NaCl, dried over K2CO3, evaporated, and chromatographed onsilica gel. Elution with hexane/AcOEt (16:1) gave i-stigmasteryl methyl ether22,23 (3.011 g, 89.9%).1H-NMR (CDCl3): 5.15 (1H. dd, J=15.2, 8.6 Hz), 5.01 (1H, dd, J=15.2, 8.6 Hz), 3.32 (3H, s), 2.77 (1H,br t, J=2.8 Hz), 2.10-2.01 (1H, m), 1.97 (1H, dt, J=12.5, 3.3 Hz), 1.89 (1H, dt, J=13.4, 3.0 Hz),1.81-1.65 (3H, m), 1.03 (3H, s), 0.91-0.78 (9H, m), 0.85 (3H, d, J=6.4 Hz), 0.73 (3H, s), 0.65 (1H, br t,J=4.7 Hz), 0.43 (1H, dd, J=8.0, 5.1 Hz). The above-mentioned i-stigmasteryl methyl ether (3.011 g,7.07 mmol) in 95% EtOH (75 mL) was hydrogenated in the presence of 10% Pd/C (500 mg) at roomtemperature. After the absorption of equimolar H2, the insoluble material was filtered off. The filtratewas concentrated under reduced pressure to give practically pure 22,23-dihydrostigmasteryl methylether22 (2.959 g, 97.8%) as a colorless foam which was used without further purification. IR (film):3058, 2956, 2868, 1464, 1382, 1201, 1183, 1098, 1015 cm-1. 1H-NMR (CDCl3): 3.32 (3H, s), 2.77(1H, t, J=2.6 Hz), 1.99 (1H, dt, J=12.5, 3.2 Hz), 1.90 (1H, dt, J=13.5, 2.9 Hz), 1.02 (3H, s), 0.96-0.79(13H, m), 0.91 (3H, d, J=6.9 Hz), 0.72 (3H, s), 0.65 (1H, br t, J=4.5 Hz), 0.43 (1H, dd, J=8.0, 5.1Hz).A mixture of the above-mentioned 22,23-dihydrostigmasteryl methyl ether (4.496 g, 10.5 mmol) andfreshly fused Zn(OAc)2 (9.25 g, 50.4 mmol) in AcOH (200 mL) was refluxed for 2 h, diluted with H2O,cooled to 0 °C, and filtered to give practically pure 22,23-dihydrostigmasteryl acetate (4.440 g, 91.9%)as colorless crystals. Recrystallization from acetone/MeOH gave analytically pure22,23-dihydrostigmasteryl acetate22,32 as colorless crystals: mp 120-121 °C (lit.22,32 mp 121-122 °C).[]D26 -39.94 (c 1.01, CHCl3) (lit.22 []D -37.5 (c 1, CHCl3)). IR (film): 2938, 2868, 1733, 1464,1373, 1244, 1033, 796, 736 cm-1. 1H-NMR (CDCl3): 5.37 (1H, br d, J=4.8 Hz), 4.65-4.55 (1H, m),2.34-2.29 (2H, m), 2.05-1.93 (2H, m), 2.03 (3H, s), 1.90-1.78 (3H, m), 1.02 (3H, s), 0.92 (3H, d, J=6.5Hz), 0.88-0.80 (9H, m), 0.68 (3H, s). A mixture of the above-mentioned 22,23-dihydrostigmasterylacetate (2.943 g, 6.45 mmol) and KOH (1.848 g, 33 mmol) in MeOH (60 mL) and Et2O (60 mL) wasstirred at room temperature for 15 h. After removal of the solvent under reduced pressure, the residuewas extracted with AcOEt, washed with H2O and saturated aq. NaCl, dried over MgSO4 and evaporatedto give 16 (2.499 g, 93.6%) as colorless crystals. Recrystallization from acetone/MeOH gaveanalytically pure 16 as colorless crystals: mp 136-138 °C (lit.22 mp 137.5-138 °C, lit.23 mp 135.5-136 °C,lit.30 mp 136.5-138 °C, lit.31 mp 139-140 °C). []D27 -33.19 (c 1.002, CHCl3) (lit.22 []D -33 (c 1,CHCl3), lit.23 []D24 -34.3 (c 1, CHCl3)). 1H-NMR (CDCl3): 5.37-5.34 (1H, m), 3.57-3.48 (1H, m),2.33-2.19 (2H, m), 2.04-1.94 (2H, m), 1.89-1.79 (3H, m), 1.01 (3H, s), 0.92 (3H, d, J=7.6 Hz), 0.88-0.80(9H, m), 0.68 (3H, s).
References:
Takahashi, Michiyasu;Hosokawa, Seiya;Ono, Yuuya;Kubodera, Noboru [Heterocycles,2016,vol. 93,# 1,p. 101 - 113]

67-56-1
780 suppliers
$9.00/25ml
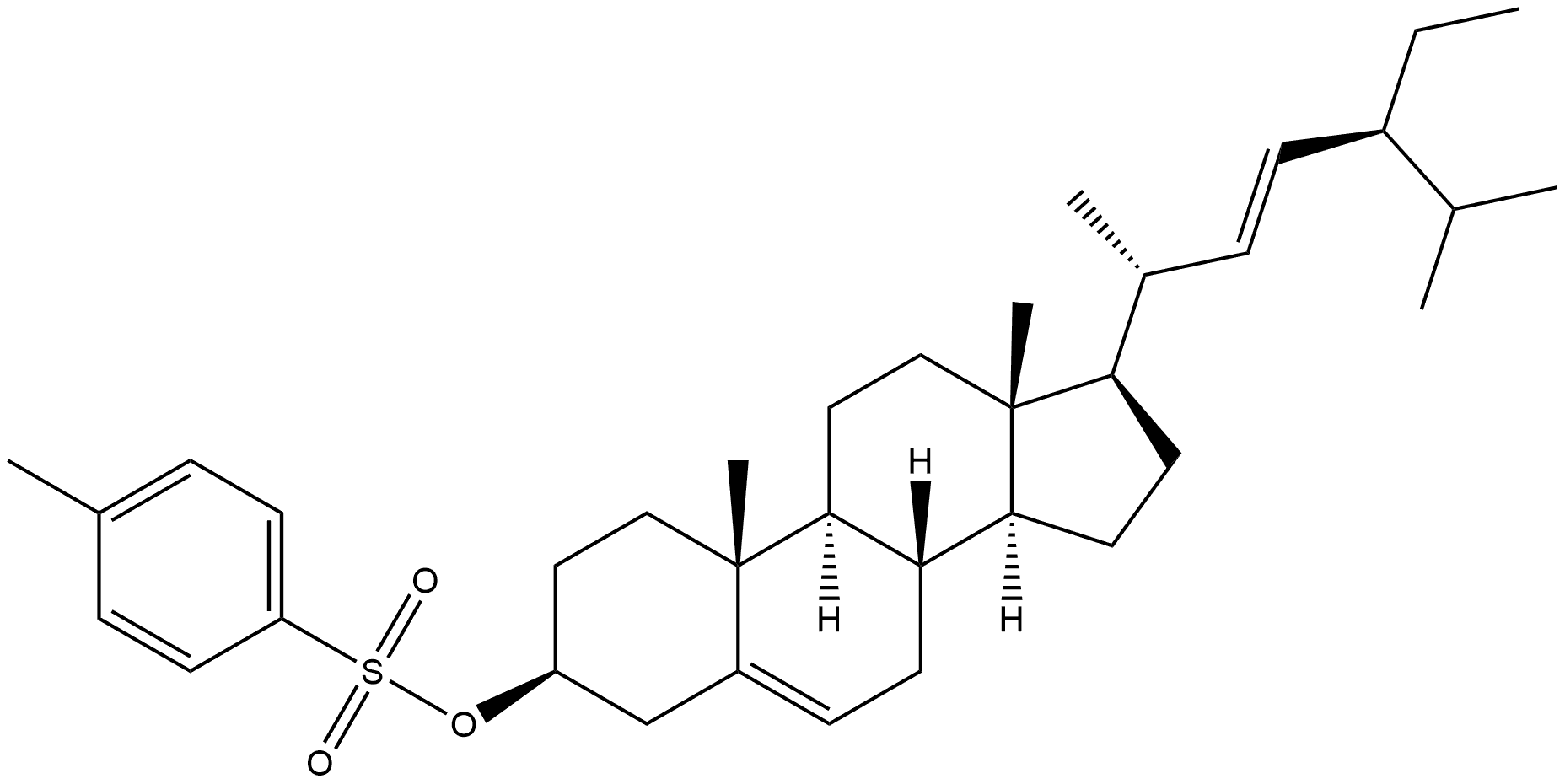
53139-42-7
3 suppliers
inquiry

10453-25-5
0 suppliers
inquiry
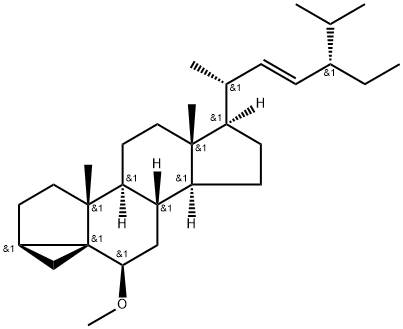
53603-94-4
5 suppliers
inquiry

32345-19-0
0 suppliers
inquiry

10453-25-5
0 suppliers
inquiry

67-56-1
780 suppliers
$9.00/25ml

53139-42-7
3 suppliers
inquiry

10453-25-5
0 suppliers
inquiry
117467-15-9
0 suppliers
inquiry