
Carbamic acid, N-[(1S)-1-(2-hydroxyethyl)pentyl]-, 1,1-dimethylethyl ester synthesis
- Product Name:Carbamic acid, N-[(1S)-1-(2-hydroxyethyl)pentyl]-, 1,1-dimethylethyl ester
- CAS Number:230637-47-5
- Molecular formula:C12H25NO3
- Molecular Weight:231.33

24424-99-5
869 suppliers
$13.50/25G
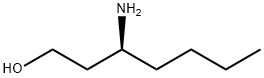
1158985-17-1
16 suppliers
inquiry
![Carbamic acid, N-[(1S)-1-(2-hydroxyethyl)pentyl]-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/230637-47-5.gif)
230637-47-5
1 suppliers
inquiry
Yield:230637-47-5 13 g
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 16 h;
Steps:
Synthesis of AA-9
Synthesis of AA-9 10119] The crude product was dissolved in dichloromethane (200 mE), triethylamine (26.17 g, 259.1 mmol) and di-tert-butyl dicarbonate (84.7 g, 194.4 mmol) was added at 0° C. The resulting mixture was stirred at room temperature for 16 hours. The mixture was partitioned between dichloromethane and watet The organic phase was washed with brine, dried and evaporated. The residue was purified by silica gel chromatography eluting with 20% ethyl acetate in petroleum ether to give AA-8 (13 g) as colorless oil.10120] ‘R NMR (400 MRz, CDC13): ? ppm 4.08-4.03 (br, 1R), 3.68 (m, 1R), 3.58-3.55 (m, 2R), 3.20-2.90 (br, 1R),1.80-1.73 (m, 1R), 1.42-1.17 (m, 15R), 0.85-0.82 (t, J=6.8 Rz, 3R).
References:
US2014/45849,2014,A1 Location in patent:Paragraph 0117; 0119; 0120
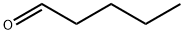
110-62-3
265 suppliers
$13.44/25ML
![Carbamic acid, N-[(1S)-1-(2-hydroxyethyl)pentyl]-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/230637-47-5.gif)
230637-47-5
1 suppliers
inquiry
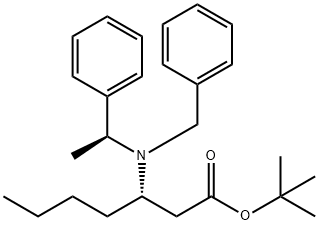
1158803-94-1
0 suppliers
inquiry
![Carbamic acid, N-[(1S)-1-(2-hydroxyethyl)pentyl]-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/230637-47-5.gif)
230637-47-5
1 suppliers
inquiry
amino]-, (3S)-](/CAS/20210305/GIF/1158803-96-3.gif)
1158803-96-3
0 suppliers
inquiry
![Carbamic acid, N-[(1S)-1-(2-hydroxyethyl)pentyl]-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/230637-47-5.gif)
230637-47-5
1 suppliers
inquiry
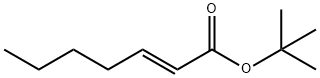
129488-82-0
4 suppliers
inquiry
![Carbamic acid, N-[(1S)-1-(2-hydroxyethyl)pentyl]-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/230637-47-5.gif)
230637-47-5
1 suppliers
inquiry