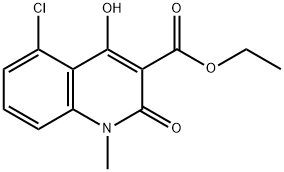
ETHYL-5-CHLORO-1,2-DIHYDRO-4-HYDROXY-1-METHYL-2-OXO-3-QUINOLINE CARBOXYLATE synthesis
- Product Name:ETHYL-5-CHLORO-1,2-DIHYDRO-4-HYDROXY-1-METHYL-2-OXO-3-QUINOLINE CARBOXYLATE
- CAS Number:248282-10-2
- Molecular formula:C13H12ClNO4
- Molecular Weight:281.69
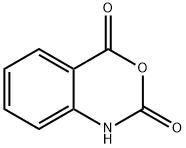
118-48-9
408 suppliers
$5.00/5G

105-53-3
729 suppliers
$5.00/25g

74-88-4
342 suppliers
$15.00/10g

248282-10-2
15 suppliers
inquiry
Yield:248282-10-2 74%
Reaction Conditions:
with hydrogenchloride in methanol;N,N-dimethyl acetamide;water;
Steps:
2 1.2Dihydro-4-hydroxy-5-chloro-1-methyl-2-oxo-quinoline-3-carboxylic acid ethyl ester
The anhydride (5 g, 0.025 mol) was dissolved in 50 ml of N,N-dimethylacetamide and cooled to 0° C. Sodium hydride (75%) (0.94 g, 0.028 mol) and then methyl iodide (1.89 ml, 0.030 mol) was added at a rate to keep the temperature below 5° C. The reaction mixture was stirred at room temperature for 5 hours. The remaining methyl iodide was removed uuder vacuum. Sodium hydride (0.94 g, 0.028 mol) was added together with diethyl malonate (4.5 g, 0.028 mol). The mixture was heated at 85° C. for 5 hours. After cooling to room temperature, 50 ml of methanol and 50 ml of 1 M hydrochloric acid and subsequently 250 ml of water were added. An emulsion was formed which crystallized on standing in a refrigerator for 72 hours. The crystalline mass was collected by filtration, washed with water, water/methanol (1:1) and heptane and dried to afford the title compound (6.3 g), yield 74%. 1H NMR (CDCl3) δ 1.46 (3H, t), 3.63 (3H, s), 4.49 (2H, q), 7.23 (1H, d), 7,27 (1H, d), 7.49 (1H, t), 15.0 (1H, s).
References:
US6133285,2000,A
![5-CHLORO-1-METHYL-1H-BENZO[D][1,3]OXAZINE-2,4-DIONE](/CAS/GIF/40707-01-5.gif)
40707-01-5
57 suppliers
$45.00/10mg

105-53-3
729 suppliers
$5.00/25g

248282-10-2
15 suppliers
inquiry
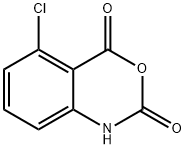
20829-96-3
82 suppliers
$20.00/100mg

105-53-3
729 suppliers
$5.00/25g

74-88-4
342 suppliers
$15.00/10g

248282-10-2
15 suppliers
inquiry

20829-96-3
82 suppliers
$20.00/100mg

248282-10-2
15 suppliers
inquiry
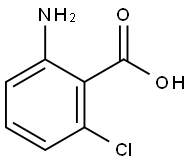
2148-56-3
362 suppliers
$5.00/10g

248282-10-2
15 suppliers
inquiry