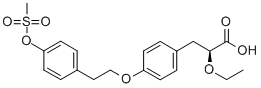
(S)-2-ETHOXY-3-[4-[2-(4-METHANESULFONYLOXY-PHENYL)-ETHOXY]-PHENYL]-PROPIONIC ACID synthesis
- Product Name:(S)-2-ETHOXY-3-[4-[2-(4-METHANESULFONYLOXY-PHENYL)-ETHOXY]-PHENYL]-PROPIONIC ACID
- CAS Number:251565-85-2
- Molecular formula:C20H24O7S
- Molecular Weight:408.47
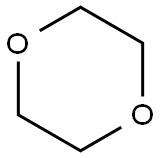
123-91-1
![Benzenepropanamide, α-ethoxy-N-[(1R)-2-hydroxy-1-phenylethyl]-4-[2-[4-[(methylsulfonyl)oxy]phenyl]ethoxy]-, (αS)-](/CAS/20211123/GIF/251565-89-6.gif)
251565-89-6
142-82-5
![(S)-2-ETHOXY-3-[4-[2-(4-METHANESULFONYLOXY-PHENYL)-ETHOXY]-PHENYL]-PROPIONIC ACID](/CAS/GIF/251565-85-2.gif)
251565-85-2
The general procedure for the synthesis of (S)-2-ethoxy-3-{4-[2-(4-methylsulfonylphenyl)ethoxy]phenyl}propionic acid from 1,4-dioxane, the compound (CAS: 251565-89-6) and n-heptane was as follows: (S)-2-ethoxy-3-{4-[2-{4-methanesulfonylphenyl)ethoxy]phenyl}propionic acid was synthesized by mixing (S)-2-ethoxy-N-(2-hydroxy(R)-1-phenylethyl)-3-[4-[2-{4-methanesulfonyloxy phenyl}ethoxy)phenyl]propionamide (4.49 g, 8.59 mmol), concentrated sulfuric acid (12.5 mL), 1,4-dioxane (50 mL), and water (50 mL) were stirred at 80 °C for 6 hours. After the reaction was completed, it was cooled to room temperature, water (100 mL) was added and the product was extracted with dichloromethane (2 x 100 mL). The organic phases were combined, washed with brine (60 mL), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The product was purified by silica gel column chromatography using gradient elution with n-heptane:ethyl acetate:acetic acid (10:10:1, v/v/v) as eluent, and finally distilled by azeotropic distillation through toluene to afford (S)-2-ethoxy-3-[4-(2-{4-methanesulfonyloxyphenyl}ethoxy)phenyl]propanoic acid (2.78 g, 79% yield). The product was structurally confirmed by 1H-NMR (600 MHz, DMSO-d6) and 13C-NMR (150 MHz, DMSO-d6).

123-91-1
710 suppliers
$16.00/25g
![Benzenepropanamide, α-ethoxy-N-[(1R)-2-hydroxy-1-phenylethyl]-4-[2-[4-[(methylsulfonyl)oxy]phenyl]ethoxy]-, (αS)-](/CAS/20211123/GIF/251565-89-6.gif)
251565-89-6
1 suppliers
inquiry
142-82-5
580 suppliers
$16.00/25ML
![(S)-2-ETHOXY-3-[4-[2-(4-METHANESULFONYLOXY-PHENYL)-ETHOXY]-PHENYL]-PROPIONIC ACID](/CAS/GIF/251565-85-2.gif)
251565-85-2
89 suppliers
$29.00/1mg
Yield: 79%
Reaction Conditions:
with sulfuric acid;acetic acid in water;ethyl acetate
Steps:
1.g g
g (S)-2-Ethoxy-3-[4-(2-{4-methanesulfonyloxyphenyl}ethoxy)phenyl]propanoic acid (S)-2-Ethoxy-N-(2-hydroxy(R)-1-phenylethyl)-3-[4-(2-{4-methanesulfonyloxyphenyl}ethoxy)phenyl]propanoic amide (4.49 g; 8.59 mmole), concentrated sulfuric acid (12.5 Ml), dioxan (50 ml) and water (50 ml) were stirred at 80° C. for 6 hours. After cooling, water (100 ml) was added and the product was extracted with dichloromethane (2*100 ml). The organic phases were combined and washed with brine (60 ml), dried (sodium sulfate), filtered and evaporated in vacuo. Purification by chromatography on silica gel using heptan:ethyl acetate:acetic acid (10:10:1) as gradient and azeotropic destillation with toluen gave 2.78 g (yield 79%) of (S)-2-ethoxy-3-[4-(2-{4-methanesulfonyloxyphenyl}ethoxy)phenyl]propanoic acid. 1H-NMR (600 MHz; DMSO-d6): δ 1.02 (t, 3H, J=7.0 Hz), 2.78 (dd, 1H, J=13.9 and 8.0 Hz), 2.86 (dd, 1H, J=13.9 and 5.2 Hz), 3.04 (t, 2H, J=6.8 Hz), 3.28 (dq, 1H, J329.1 and 7.0 Hz), 3.35 (s, 3H), 3.49 (dq, 1H, J=9.1 and 7.0 Hz), 3.92 (dd, 1H, J=5.2 and 7.7 Hz),4.15(t, 2H, J=6.8 Hz), 6.82 (d, 2H, J=8.7 Hz), 7.11 (d, 2H, J=8.7 Hz), 7.27 (d, 2H, J=8.5 Hz), 7.42 (d, 2H, J=8.5 Hz), 12.59 (s, br, 1 OH). 13C-NMR (150 MHz; DMSO-d6): δ 15.2, 34.4, 37.5, 37.7, 65.0, 67.9, 79.4, 114.2, 122.2, 129.6, 130.4, 130.7, 138.0, 147.8, 157.1, 173.4.
References:
AstraZeneca AB US6258850, 2001, B1
![(S)-2-Ethoxy-3-[4-(2-{4-Methanesulfonyloxyphenyl}ethoxy)phenyl]propanoic acid ethyl ester](/CAS/20150408/GIF/251565-91-0.gif)
251565-91-0
1 suppliers
inquiry
![(S)-2-ETHOXY-3-[4-[2-(4-METHANESULFONYLOXY-PHENYL)-ETHOXY]-PHENYL]-PROPIONIC ACID](/CAS/GIF/251565-85-2.gif)
251565-85-2
89 suppliers
$29.00/1mg