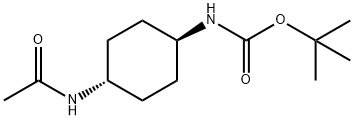
tert-Butyl (1R*,4R*)-4-acetamidocyclohexylcarbamate synthesis
- Product Name:tert-Butyl (1R*,4R*)-4-acetamidocyclohexylcarbamate
- CAS Number:251947-21-4
- Molecular formula:C13H24N2O3
- Molecular Weight:256.34
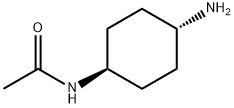
873537-23-6
14 suppliers
$167.00/500MG

24424-99-5
871 suppliers
$13.50/25G

251947-21-4
6 suppliers
$469.00/1g
Yield:251947-21-4 93.4%
Reaction Conditions:
with sodium hydroxide in 1,4-dioxane;lithium hydroxide monohydrate at 20; for 24 h;Inert atmosphere;Solvent;
Steps:
1.2; 2.2; 3.2 Step 2:
Add 128.61g of Intermediate 1 and 188.65g of (Boc)2O to 500mL of 1,4-dioxane, under nitrogen protection, slowly add a saturated aqueous solution of 49.41g of NaOH to the system, and react at room temperature for 24h, add dilute hydrochloric acid after the reaction to adjust the pH to be weakly acidic, add dichloromethane to extract the reaction system, the organic phase is evaporated and concentrated, and after recrystallization, 196.25g of intermediate 2 is obtained by filtration, and the yield is 93.4%
References:
CN114835610,2022,A Location in patent:Paragraph 0071-0076

75-36-5
589 suppliers
$17.92/100G
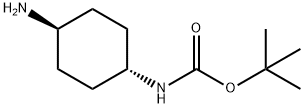
177906-48-8
216 suppliers
$6.00/250mg

251947-21-4
6 suppliers
$469.00/1g

108-24-7
0 suppliers
$14.00/250ML

177906-48-8
216 suppliers
$6.00/250mg

251947-21-4
6 suppliers
$469.00/1g
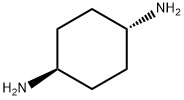
2615-25-0
281 suppliers
$5.00/1g

251947-21-4
6 suppliers
$469.00/1g