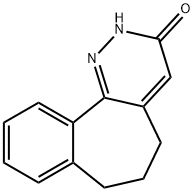
6,7-dihydro-2H-benzo[6,7]cyclohepta[1,2-c]pyridazin-3(5H)-one synthesis
- Product Name:6,7-dihydro-2H-benzo[6,7]cyclohepta[1,2-c]pyridazin-3(5H)-one
- CAS Number:25823-52-3
- Molecular formula:C13H12N2O
- Molecular Weight:212.25
![4a,5,6,7-tetrahydro-2H-benzo[6,7]cyclohepta[1,2-c]pyridazin-3(4H)-one](/CAS/20180529/GIF/25742-87-4.gif)
25742-87-4
![6,7-dihydro-2H-benzo[6,7]cyclohepta[1,2-c]pyridazin-3(5H)-one](/CAS/20180601/GIF/25823-52-3.gif)
25823-52-3
Using 4a,5,6,7-tetrahydro-2H-benzo[6,7]cyclohepta[c]pyridazin-3(4H)-one (4.7 g, 22 mmol) as starting material, it was mixed with anhydrous copper(II) chloride (6 g, 44 mmol) in acetonitrile (45 mL) and the reaction was carried out at reflux for 2 hours. After completion of the reaction, the mixture was cooled to room temperature and subsequently poured into ice water (200 g). The resulting solid was washed sequentially by 10% hydrochloric acid solution (~20 mL x 2) and cold water (~20 mL x 2). After freeze-drying treatment, the target product 3-oxo-6,7-dihydro-5H-benzo[6,7]cyclohepta[1,2-c]pyridazine (4.2 g, 90% yield) was obtained as a white solid. The product was characterized by 1H NMR (300 MHz, CDCl3): δ 10.80 (broad single peak, 1H), 7.53-7.21 (multiple peaks, 4H), 6.77 (single peak, 1H), 2.66 (triple peak, J = 6.9 Hz, 2H), 2.45 (triple peak, J = 6.9 Hz, 2H), 2.14 (quadruple peak, J = 6.9 Hz. 2H).LC-MS analysis showed 100% purity, mass spectrum (m/e): 213 (MH+).
![4a,5,6,7-tetrahydro-2H-benzo[6,7]cyclohepta[1,2-c]pyridazin-3(4H)-one](/CAS/20180529/GIF/25742-87-4.gif)
25742-87-4
16 suppliers
inquiry
![6,7-dihydro-2H-benzo[6,7]cyclohepta[1,2-c]pyridazin-3(5H)-one](/CAS/20180601/GIF/25823-52-3.gif)
25823-52-3
29 suppliers
$73.00/100mg
Yield:25823-52-3 90%
Reaction Conditions:
with copper dichloride in acetonitrile; for 2 h;Product distribution / selectivity;Heating / reflux;
Steps:
7.A
SYNTHETIC PREPARATION 7; Synthesis of 3-oxo-6,7-dihydro-5/-/-benzo[6,7]cyclohepta[1 ,2-c]pyridazineCompound of formula (De)A. A mixture of the compound of formula (Dd), 4a,5,6,7-tetrahydro-2H- benzo[6,7]cyclohepta[c]pyridazin-3(4H)-one (4.7 g, 22 mmol) and anhydrous copper(ll) chloride (6 g, 44 mmol) was refluxed in acetonitrile (45 mL) for 2 hours. After cooling to ambient temperature, the mixture was poured into ice-water (200 g) and the solid obtained was washed with 10% HCI solution twice (about 20 mLx2) and cold water twice (about 20 mLx2). After freeze-drying, the compound of formula (De), 3-oxo-6,7- dihydro-5H-benzo[6,7]cyclohepta[1 ,2-c]pyridazine (4.2 g, 90%) was obtained as a white solid, 1H NMR (300 MHz, CDCI3) δ: 10.80 (bs, 1 H), 7.53-7.21 (m, 4H), 6.77 (s, 1 H), 2.66 (t, J = 6.9 Hz, 2H), 2.45 (t, J = 6.9 Hz, 2H), 2.14 (quant, J = 6.9 Hz, 2H); LC- MS: purity: 100%; MS (m/e) : 213 (MH+).
References:
WO2008/83353,2008,A1 Location in patent:Page/Page column 126
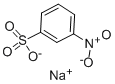
127-68-4
575 suppliers
$5.00/25g
![4a,5,6,7-tetrahydro-2H-benzo[6,7]cyclohepta[1,2-c]pyridazin-3(4H)-one](/CAS/20180529/GIF/25742-87-4.gif)
25742-87-4
16 suppliers
inquiry
![6,7-dihydro-2H-benzo[6,7]cyclohepta[1,2-c]pyridazin-3(5H)-one](/CAS/20180601/GIF/25823-52-3.gif)
25823-52-3
29 suppliers
$73.00/100mg
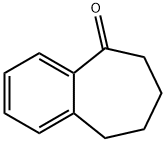
826-73-3
158 suppliers
$15.00/1g
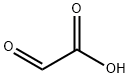
298-12-4
475 suppliers
$5.00/25g
![6,7-dihydro-2H-benzo[6,7]cyclohepta[1,2-c]pyridazin-3(5H)-one](/CAS/20180601/GIF/25823-52-3.gif)
25823-52-3
29 suppliers
$73.00/100mg

826-73-3
158 suppliers
$15.00/1g
![6,7-dihydro-2H-benzo[6,7]cyclohepta[1,2-c]pyridazin-3(5H)-one](/CAS/20180601/GIF/25823-52-3.gif)
25823-52-3
29 suppliers
$73.00/100mg

25742-81-8
1 suppliers
inquiry
![6,7-dihydro-2H-benzo[6,7]cyclohepta[1,2-c]pyridazin-3(5H)-one](/CAS/20180601/GIF/25823-52-3.gif)
25823-52-3
29 suppliers
$73.00/100mg