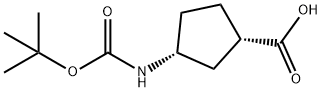
(+)-(1S,3R)-N-BOC-3-AMINOCYCLOPENTANECARBOXYLIC ACID synthesis
- Product Name:(+)-(1S,3R)-N-BOC-3-AMINOCYCLOPENTANECARBOXYLIC ACID
- CAS Number:261165-05-3
- Molecular formula:C11H19NO4
- Molecular Weight:229.27
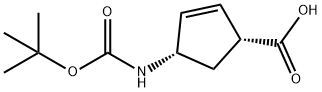
151907-80-1

261165-05-3
The general procedure for the synthesis of (1S,3R)-3-((tert-butoxycarbonyl)amino)cyclopent-2-enecarboxylic acid using (1R,4S)-4-((tert-butoxycarbonyl)amino)cyclopent-2-enecarboxylic acid as a starting material was as follows: (1R,4S)-4-((tert-butoxycarbonyl)amino)cyclopent-2-enecarboxylic acid (230 g, 1.0 mol) prepared in step A was hydrogenated with 10% Pd/ C catalyst (5.0 g) in 500 mL of methanol and hydrogenated on a Parr shaker at 50 psi hydrogen pressure for 1 hour. Upon completion of the reaction, the catalyst was removed by filtration followed by evaporation of the filtrate to remove the solvent. The resulting residue was dissolved in dichloromethane and dried by adding anhydrous sodium sulfate. After filtration again, the filtrate was evaporated and dried under vacuum to afford (1S,3R)-3-((tert-butoxycarbonyl)amino)cyclopentanecarboxylic acid as a light yellow solid (230 g, 99% yield). Analyzed by LC-MS, the calculated value of [M+H+] for C11H19NO4 was 230 and the measured value was 230, which was as expected.

24424-99-5
868 suppliers
$13.50/25G
71830-07-4
122 suppliers
$55.00/100 mg

261165-05-3
151 suppliers
$39.00/250mg
Yield: 99%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in 1,4-dioxane;water at 20 - 27; for 3 h;
Steps:
21 Typical Procedure for the Preparation of 1,2,4-Ox- adiazoles as Exemplified by the Preparation ofIntermediate 126, (1 R,35)-3-(3-methyl-1 ,2,4-oxadi- azol-5-yl)cyclopentanamine hydrochloride salt
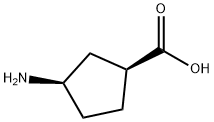
di-tert-l3utyl dicarbonate (1.25 g, 5.75 mmol) and DIPEA (2.61 mE, 15.0 mmol) were added to a solution of (1 S,3R)-3-aminocyclopentanecarboxylic acid (0.646 g, 5.0 mmol) in 1,4-dioxane (5 mE) and water (5 mE) and the resulting mixture was stirred at RT for 3 h. The reaction mixture was acidified to pH 2 using 1 M aqueous HC1 and extracted with DCM (x4). The combined organic extractswere passed through a phase separator cartridge and con-centrated in-vacuo to give (1 S,3R)-3-[(tert-butoxycarbonyl) amino]cyclopentanecarboxylic acid (1.13 g, 99%). ‘H NMR (400 MHz, CDC13) ö: 1.44 (s, 9H),1.56-2.06 (m, 5H), 2.16-2.33 (m, 1H), 2.79-2.93 (m, 1H),3.87-4.18 (m, 1H), 4.86 (bt s., 1H). One exchangeable proton not observed.
References:
Heptares Therapeutics Limited;Brown, Giles Albert;Congreve, Miles Stuart;Pickworth, Mark;Tehan, Benjamin Gerald US2018/105491, 2018, A1 Location in patent:Paragraph 0567; 0773; 0774; 0775; 0776

151907-80-1
166 suppliers
$22.00/250mg

261165-05-3
151 suppliers
$39.00/250mg
![(1S,3R)-Methyl 3-[(tert-butoxycarbonyl)-amino]cyclopentanecarboxylate](/CAS/GIF/554451-12-6.gif)
554451-12-6
15 suppliers
inquiry

261165-05-3
151 suppliers
$39.00/250mg
![2-Azabicyclo[2.2.1]heptane-2-carboxylic acid, 3-oxo-, 1,1-dimethylethyl ester, (1R,4S)-](/CAS/20210111/GIF/693245-53-3.gif)
693245-53-3
3 suppliers
inquiry

261165-05-3
151 suppliers
$39.00/250mg
![2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/49805-30-3.gif)
49805-30-3
382 suppliers
$5.00/5g

261165-05-3
151 suppliers
$39.00/250mg