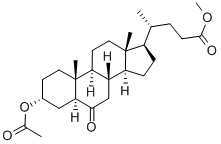
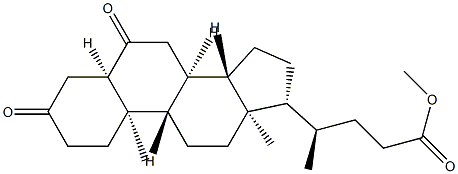
3-ALPHA-HYDROXY-6-OXO-5-ALPHA-CHOLAN-24-OIC ACID METHYL ESTER 3-ACETATE synthesis
- Product Name:3-ALPHA-HYDROXY-6-OXO-5-ALPHA-CHOLAN-24-OIC ACID METHYL ESTER 3-ACETATE
- CAS Number:2616-79-7
- Molecular formula:C27H42O5
- Molecular Weight:446.62
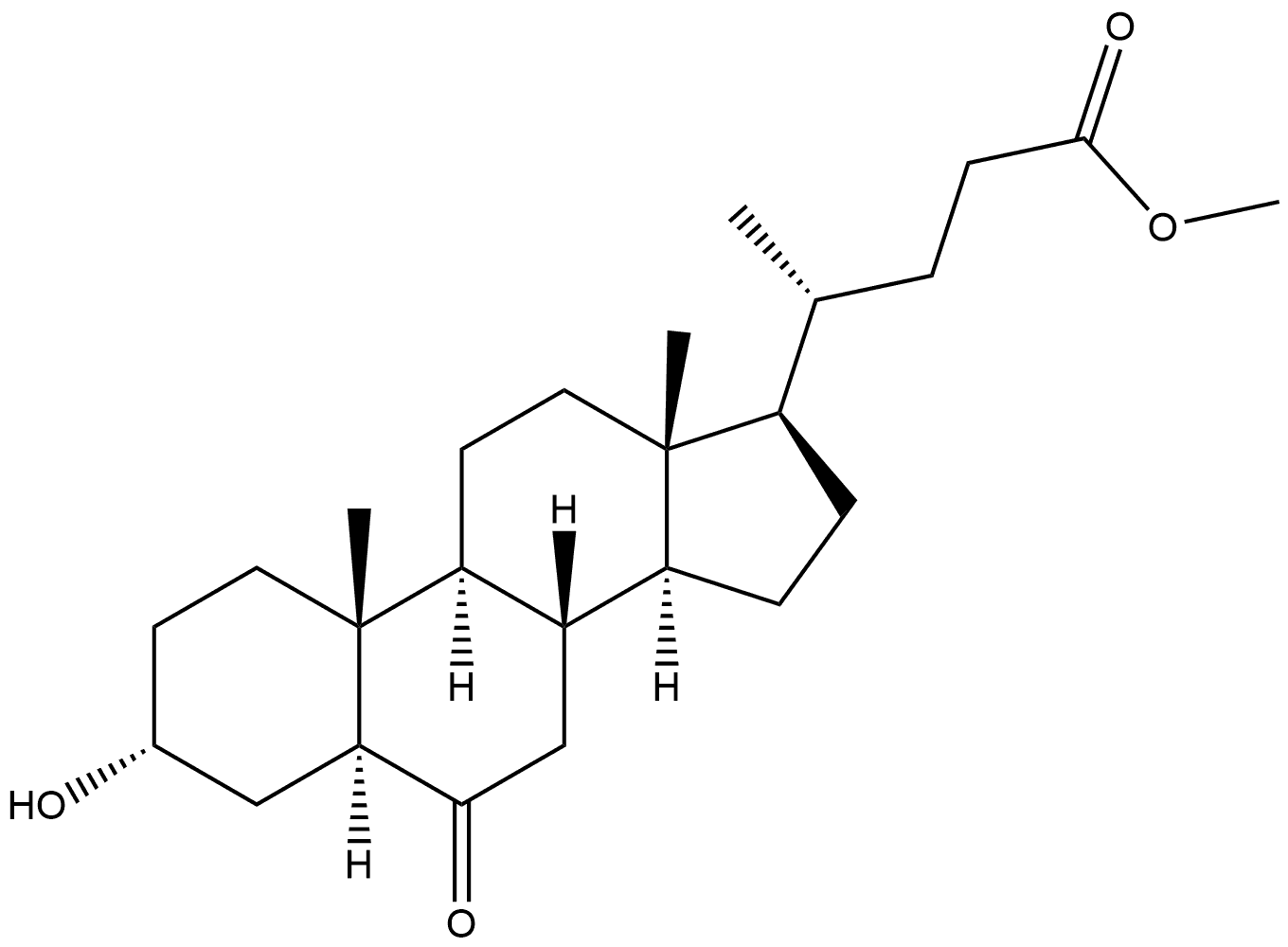
34186-19-1
2 suppliers
inquiry

108-24-7
0 suppliers
$14.00/250ML

2616-79-7
21 suppliers
$313.00/500mg
Yield:2616-79-7 95%
Reaction Conditions:
with pyridine;dmap in dichloromethane at 20; for 0.5 h;
Steps:
Methyl 3α-acetoxy-6-oxo-5α-cholan-24-oate (12)
To a solution of compound 8 (2.00g, 4.94 mmol) in 20 mLof DCM, 5 mg of DMAP, 1 mL of pyridine and 0.5 mL (5.3 mmol) of Ac2O were added. The reactionmixture was kept under constant stirring and room temperature for 30 min. The end of reactionwas verified by TLC, the mixture was then concentrated to a volume approximately 5 mL underreduced pressure. Then AcOEt (30 mL) were added. The organic layer was washed with water(2 x15 mL), dried over Na2SO4, and filtered. The solvent was evaporated under reduced pressure andthe crude was re-dissolved in CH2Cl2 (5 mL) and chromatographed on silica gel with EtOAc/hexanemixtures of increasing polarity (0.2:9.8 →4.7:5.3). Compound 12 (1.9 g, 95% yield) was a colorlesssolid (m.p. = 174-176 °C, MeOH/Et2O) IR (cm1): 2943 (CH3); 2869 (CH2); 1737 (C=O); 1708 (C=O);1435 (CH2); 1376 (CH3); 1262 (C-O); 1221 (C-O); 1172 (C-O). 1H-NMR: 5.12 (m, 1H, H-3); 3.66 (s, 3H,CH3O); 2.56 (dd, J = 3.3 and 12.0 Hz, 1H, H-5); 2.38 (ddd, J = 5.4, 10.3 and 15.4 Hz, 1H, H-23); 2.04 (s,3H, CH3CO); 0.94 (d, J = 6.4 Hz, 3H, H-21); 0.74 (s, 3H, H-19); 0.67 (s, 3H, H-18). 13C-NMR: See Table 1.1H-NMR and 13C-NMR are shown in Supplementary Materials.
References:
Herrera, Heidy;Carvajal, Rodrigo;Olea, Andrés F.;Espinoza, Luis [Molecules,2016,vol. 21,# 9,art. no. 1139]
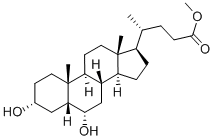
2868-48-6
79 suppliers
$54.60/1g

2616-79-7
21 suppliers
$313.00/500mg
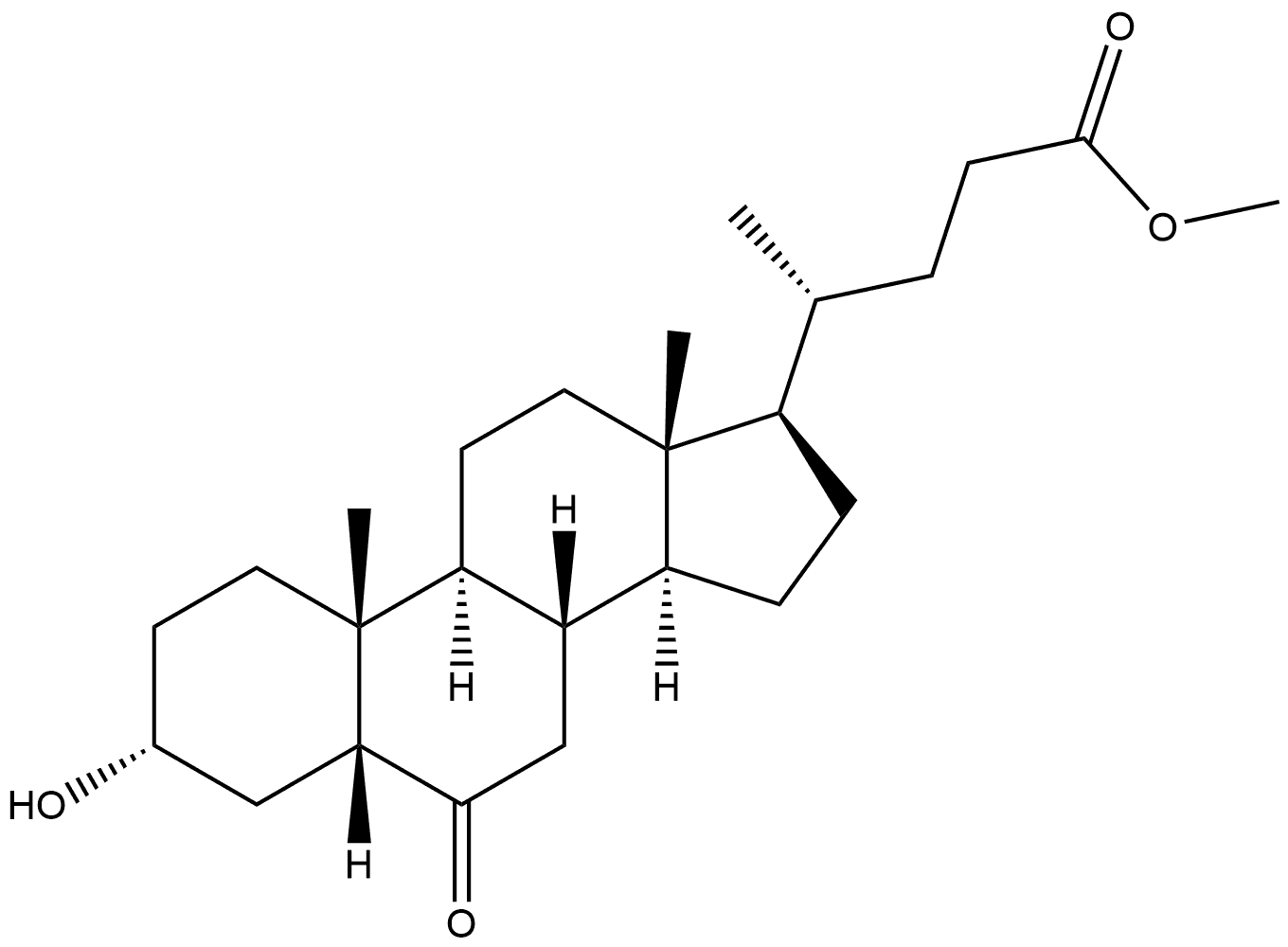
2862-62-6
2 suppliers
inquiry

2616-79-7
21 suppliers
$313.00/500mg
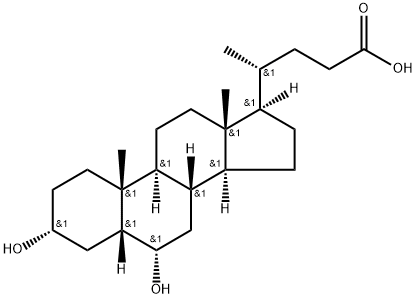
83-49-8
472 suppliers
$29.00/25g

2616-79-7
21 suppliers
$313.00/500mg
1175-04-8
0 suppliers
inquiry

2616-79-7
21 suppliers
$313.00/500mg