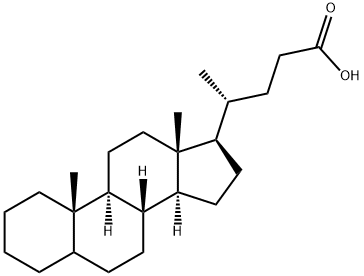
CHOLANIC ACID synthesis
- Product Name:CHOLANIC ACID
- CAS Number:25312-65-6
- Molecular formula:C24H40O2
- Molecular Weight:360.57
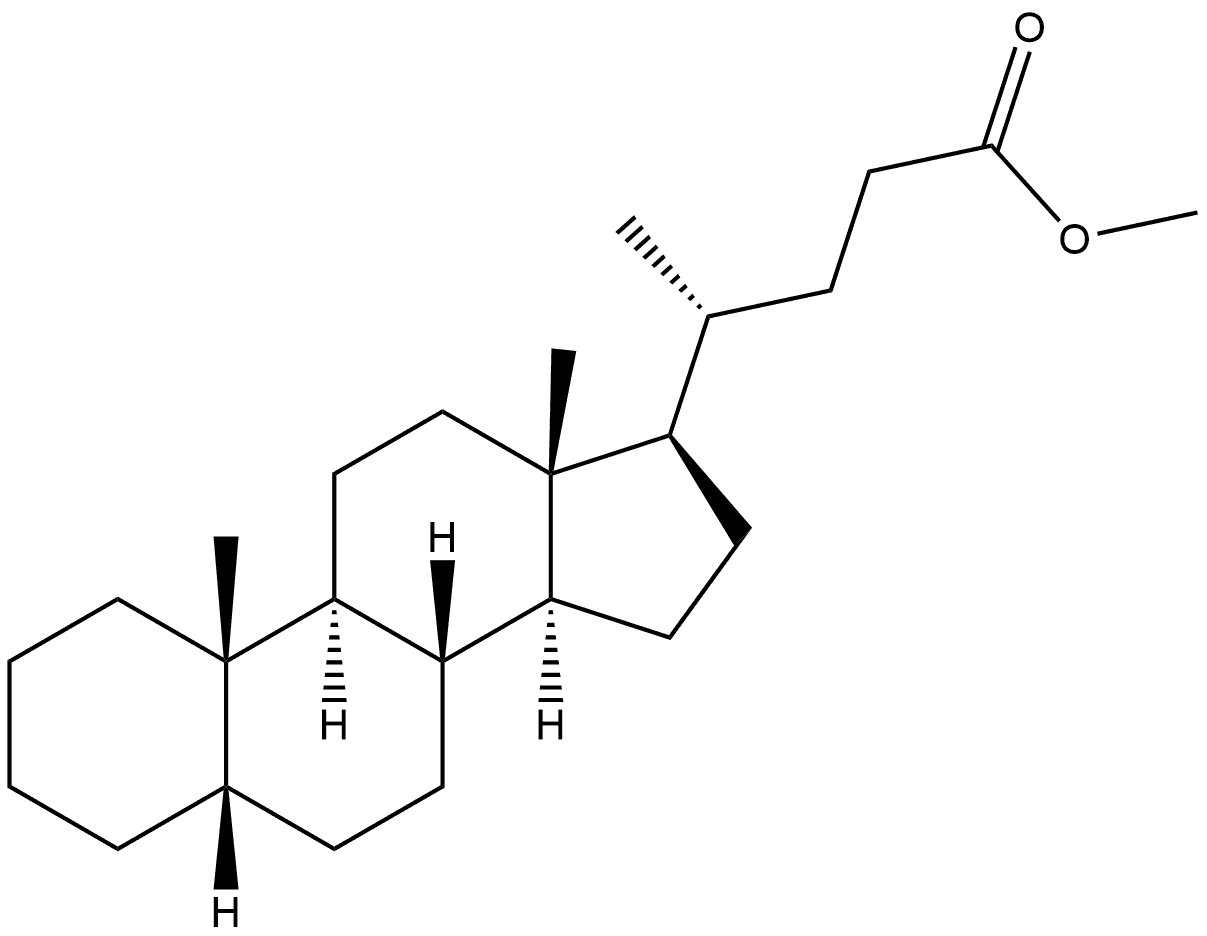
2204-14-0
4 suppliers
inquiry

25312-65-6
7 suppliers
inquiry
Yield:25312-65-6 100%
Reaction Conditions:
with sodium hydroxide in methanol;water;Inert atmosphere;Reflux;
Steps:
2.6 5β-cholan-24-oic acid (3)
Compound 13 (500 mg, 1.33 mmol) was hydrolyzed with a methanol solution of sodium hydroxide (5%, 5 mL) in H2O (5 mL) overnight under reflux. The resulting solution was then concentrated under vacuum, diluted with water, acidified with HCl 6 N and extracted with ethyl acetate (3 * 50 mL). The collected organic phases were washed with brine, dried over Na2SO4 anhydrous and evaporated under reduced pressure to give 480 mg of compound 3 (quantitative yield). An analytic sample was obtained by HPLC on a Nucleodur 100-5 C18 (5 μm; 10 mm i.d. * 250 mm) with MeOH/H2O (999.5:0.5) as eluent (flow rate 3 mL/min, tR = 21 min); [α]25D = +13.3 (c 0.32, CHCl3); selected 1H NMR (400 MHz CDCl3): δ 2.39 (1H, m), 2.26 (1H, m), 0.93 (3H, d, J = 6.6 Hz), 0.91 (3H, s), 0.66 (3H, s). 13C NMR (100 MHz CDCl3): δ 179.1, 56.8, 56.2, 50.6, 43.9, 43.0, 40.7, 40.5, 37.8, 36.1 (2C), 35.6 (2C), 31.1, 28.4, 27.7, 27.5, 27.3, 26.8, 24.5, 21.6, 21.0, 18.5, 12.3. HR ESIMS m/z 359.2956 [M-H]-, C24H39O2 requires 359.2950.
References:
Sepe, Valentina;Renga, Barbara;Festa, Carmen;Finamore, Claudia;Masullo, Dario;Carino, Adriana;Cipriani, Sabrina;Distrutti, Eleonora;Fiorucci, Stefano;Zampella, Angela [Steroids,2016,vol. 105,p. 59 - 67]
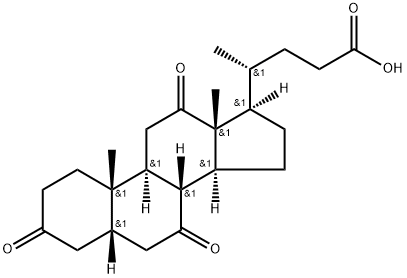
81-23-2
288 suppliers
$31.00/1g

25312-65-6
7 suppliers
inquiry
2958-05-6
21 suppliers
inquiry

25312-65-6
7 suppliers
inquiry
4057-84-5
26 suppliers
inquiry

25312-65-6
7 suppliers
inquiry
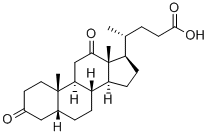
434-13-9
368 suppliers
$10.00/1g

25312-65-6
7 suppliers
inquiry