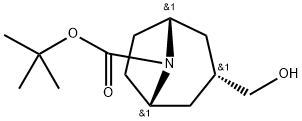
8-Azabicyclo[3.2.1]octane-8-carboxylic acid, 3-(hydroxymethyl)-, 1,1-dimethylethyl ester, (3-endo)- synthesis
- Product Name:8-Azabicyclo[3.2.1]octane-8-carboxylic acid, 3-(hydroxymethyl)-, 1,1-dimethylethyl ester, (3-endo)-
- CAS Number:273376-39-9
- Molecular formula:C13H23NO3
- Molecular Weight:241.33
![8-Azabicyclo[3.2.1]octane-3-methanol, 8-(phenylmethyl)-, (3-endo)-](/CAS/20210305/GIF/852460-98-1.gif)
852460-98-1
0 suppliers
inquiry

24424-99-5
869 suppliers
$13.50/25G
![8-Azabicyclo[3.2.1]octane-8-carboxylic acid, 3-(hydroxymethyl)-, 1,1-dimethylethyl ester, (3-endo)-](/CAS/20211123/GIF/273376-39-9.gif)
273376-39-9
25 suppliers
$268.00/250mg
Yield: 81%
Reaction Conditions:
Stage #1:[(3-endo)-8-(phenylmethyl)-8-azabicyclo[3.2.1]oct-3-yl]methanol with hydrogenchloride;hydrogen;palladium hydroxide on carbon in ethanol;water at 20; under 2844.39 Torr; for 66 h;
Stage #2:di-tert-butyl dicarbonate with sodium hydroxide in 1,3-dioxane;water at 20;
Steps:
A solution of (3-endo)- (8-benzyl-8-azabicyclo [3.2. 1] oct-3-yl) methanol (2.31 g, 10 mmol) in ethanol (45 ml) and 6N HCI (2 ml) containing palladium hydroxide on carbon (Pearlman's catalyst, 2.6 g, 20% (w/w) ) was hydrogenated (55 psi H2) at room temperature for 18 h. After filtration of the catalyst, the filtrate was concentrated under vacuum. The residue was redissolved in EtOH (45 ml) and 6N HCI (2ml), to which palladium hydroxide on carbon (2.6 g) was added. The reaction mixture was hydrogenated (55 psi H2) at room temperature for 2 days. The catalyst was filtered off over Celite and the filtrate was evaporated. The residue and di-tert-butyl dicarbonate (3.2 g, 15 mmol) were dissolved in 60 ml of dioxane : 1 N NaOH (2: 1) and stirred overnight at room temperature. The solvent was evaporated and the residue partitioned between ethyl acetate (3 X 50 ml) and water (50 ml). The combined organic layers were dried over Na2SO4 and evaporated. The residual oil was purified by loading onto an aminopropyl SPE cartridge (30g) and eluting with DCM (4 X 30 ml), EA (4 X 30 ml) and MeOH (2 X 30 ml). The DCM fractions were combined and evaporated to give a colourless oil (1.95 g, 81 %). LC/MS: 1.65 min (100 %), MH+: 242. NMR (CDCI3) : 4.15 ppm (broad, 2H), 3.64 ppm (d ; 2H), 2.20 ppm (broad, 2H), 1.97 ppm (broad, 2H), 1.85 ppm (m, 1 H), 1.60 ppm (m, 2H), 1.40-1. 50 ppm (s+broad, 11 H)
References:
GLAXO GROUP LIMITED WO2005/46586, 2005, A2 Location in patent:Page/Page column 24
![(1R,3r,5S)-8-Azabicyclo[3.2.1]octan-3-ylmethanol](/CAS/20200119/GIF/1257512-65-4.gif)
1257512-65-4
3 suppliers
inquiry

24424-99-5
869 suppliers
$13.50/25G
![8-Azabicyclo[3.2.1]octane-8-carboxylic acid, 3-(hydroxymethyl)-, 1,1-dimethylethyl ester, (3-endo)-](/CAS/20211123/GIF/273376-39-9.gif)
273376-39-9
25 suppliers
$268.00/250mg
185099-67-6
246 suppliers
$5.00/250mg
![8-Azabicyclo[3.2.1]octane-8-carboxylic acid, 3-(hydroxymethyl)-, 1,1-dimethylethyl ester, (3-endo)-](/CAS/20211123/GIF/273376-39-9.gif)
273376-39-9
25 suppliers
$268.00/250mg
![exo-8-Boc-8-azabicyclo[3.2.1]octane-3-Methanol](/CAS/20150408/GIF/273207-58-2.gif)
273207-58-2
34 suppliers
$179.00/250 mg

185099-67-6
246 suppliers
$5.00/250mg
![8-Azabicyclo[3.2.1]octane-8-carboxylic acid, 3-(hydroxymethyl)-, 1,1-dimethylethyl ester, (3-endo)-](/CAS/20211123/GIF/273376-39-9.gif)
273376-39-9
25 suppliers
$268.00/250mg