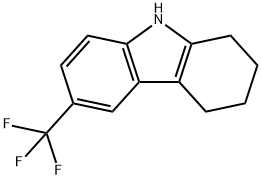
3-(Trifluoromethyl)-6,7,8,9-tetrahydro-5H-carbazole synthesis
- Product Name:3-(Trifluoromethyl)-6,7,8,9-tetrahydro-5H-carbazole
- CAS Number:2805-84-7
- Molecular formula:C13H12F3N
- Molecular Weight:239.24
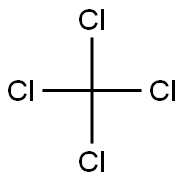
108-94-1
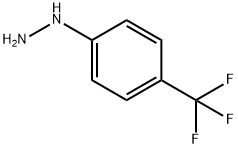
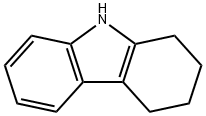
368-90-1

2805-84-7
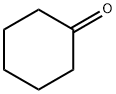
16A. Synthesis of 6-(trifluoromethyl)-2,3,4,4a,9,9a-hexahydro-1H-carbazole To a solution of 4-(trifluoromethyl)phenylhydrazine (4.71 g, 26.74 mmol) in ethanol (20 mL) was added concentrated hydrochloric acid (10 drops) followed by a solution of cyclohexanone (2.62 g, 26.74 mmol) in ethanol (4 mL). The reaction mixture was stirred at room temperature for 45 minutes and subsequently concentrated under vacuum. The resulting solid was dissolved in a mixture of acetic acid (21 mL) and concentrated sulfuric acid (3.5 mL). The reaction mixture was heated at 85 °C for 30 min, cooled to room temperature and poured into ice water. The suspension formed was filtered and the solid was dried to give 6-(trifluoromethyl)-2,3,4,9-tetrahydro-1H-carbazole as a brown solid (6.01 g, 94% yield). Mass spectrum (electrospray positive ion mode) m/e 240 [M + H]+.

108-94-1
583 suppliers
$12.00/50g

368-90-1
160 suppliers
$15.00/1g

2805-84-7
37 suppliers
$24.00/250mg
Yield:2805-84-7 94%
Reaction Conditions:
Stage #1: cyclohexanone;4-(trifluoromethyl)phenylhydrazinewith hydrogenchloride in ethanol at 20; for 0.75 h;
Stage #2: with sulfuric acid;acetic acid at 85; for 0.5 h;
Steps:
16.a
16a. 6-(Trifluoromethyl)-2,3,4,4a,9,9a-hexahydro-1H-carbazole To (4-trifluoromethyl-phenyl)-hydrazine (4.71 g, 26.74 mmol) in ethanol (20 mL) was added concentrated hydrochloric acid (10 drops) followed by a solution of cyclohexanone (2.62 g, 26.74 mmol) in ethanol (4 mL). The reaction was stirred at ro temperature for 45 minutes and concentrated in vacuo. The resulting solid was dissolved in a mixture of acetic acid (21 mL) and concentrated sulfuric acid (3.5 mL). The reaction was heated at 85° C. for 30 minutes, cooled to room temperature, and poured into ice water. The resulting suspension was filtered and dried to give the title compound as a tan solid (6.01 g, 94%). MS (ES+) m/e 240 [M+H]+.
References:
US2009/170882,2009,A1 Location in patent:Page/Page column 14

110-52-1
567 suppliers
$9.00/1g
100846-24-0
170 suppliers
$35.00/250mg

2805-84-7
37 suppliers
$24.00/250mg
![Diazene, 1,2-bis[4-(trifluoromethyl)phenyl]-](/CAS/20210305/GIF/34913-29-6.gif)
34913-29-6
5 suppliers
inquiry

108-94-1
583 suppliers
$12.00/50g

2805-84-7
37 suppliers
$24.00/250mg

108-94-1
583 suppliers
$12.00/50g
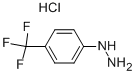
2923-56-0
187 suppliers
$8.00/5g

2805-84-7
37 suppliers
$24.00/250mg