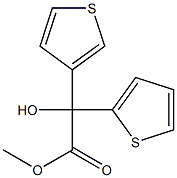
Methyl 2-Hydroxy-2-(Thiophen-2-Yl)-2-(Thiophen-3-Yl)Acetate synthesis
- Product Name:Methyl 2-Hydroxy-2-(Thiophen-2-Yl)-2-(Thiophen-3-Yl)Acetate
- CAS Number:28748-67-6
- Molecular formula:C11H10O3S2
- Molecular Weight:254.3253
1003-09-4
484 suppliers
$10.00/25g

104749-67-9
16 suppliers
$45.00/10mg

28748-67-6
20 suppliers
$270.00/50mg
Yield:28748-67-6 68%
Reaction Conditions:
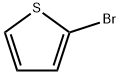
Stage #1: 2-bromothiophenewith n-butyllithium in tetrahydrofuran; for 0.0833333 h;Inert atmosphere;Cooling;
Stage #2: methyl 2-oxo-2-(thiophen-3-yl)acetate in tetrahydrofuran; for 0.5 h;Inert atmosphere;Cooling;
Steps:
Lithiation of 2-Bromothiophene: preparation of oxo-acetate (4a,c) and glycolates (1a,b).
General procedure: In a flame-dried round flask, organolithium reagent (0.95 mmol) was added dropwise to a solution of 2-bromothiophene 1b (193 mg, 1.0 mmol) in anhydrous THF (15 mL) under N2 at -80 °C. After 20 min, a THF (5.0 mL) solution of oxalate 3 or oxo-acetate 4a-c was added. After the disappearing of the starting thiophene, the reaction was quenched by saturated aqueous NH4Cl and extracted with AcOEt (2×20 mL); the collected organic phases were washed with brine (1×10 mL), dried over Na2SO4 and the solvent was evaporated under vacuum (RV). The resulting crude was purified by FCC - AcOEt/hexane (1:9) - on silica gel. Yield, physical, spectroscopic and analytical data of products 4a,c, 1a,b are as follows.
References:
Foschi, Francesca;Bonandi, Elisa;Mereu, Andrea;Pacchetti, Barbara;Gozzini, Davide;Passarella, Daniele [Arkivoc,2018,vol. 2018,# 7,p. 423 - 430]