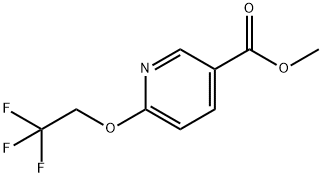
6-(2,2,2-TRIFLUOROETHOXY)NICOTINIC ACID METHYL ESTER synthesis
- Product Name:6-(2,2,2-TRIFLUOROETHOXY)NICOTINIC ACID METHYL ESTER
- CAS Number:287979-27-5
- Molecular formula:C9H8F3NO3
- Molecular Weight:235.16
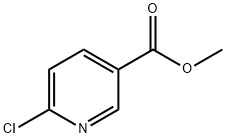
73781-91-6
287 suppliers
$5.00/1g
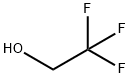
75-89-8
488 suppliers
$9.00/1g

287979-27-5
36 suppliers
$320.31/1g
Yield:-
Reaction Conditions:
Stage #1: 2,2,2-trifluoroethanolwith sodium hydride in tetrahydrofuran;mineral oil at 20; for 0.5 h;
Stage #2: 6-Chloronicotinic acid methyl ester in tetrahydrofuran;acetonitrile;mineral oil at 70; for 1.5 h;
Steps:
To a stirred solution of 2,2,2-trifluoroethanol (1.0 mL, 14 mmol) in THF (25 mL) was added sodium hydride (60% dispersion in oil, 0.56 g, 14 mmol). The reaction mixture was stirred at r.t. for 30 minutes prior to the addition of 6-chloronicotinic acid methyl ester (1.71 g, 10 mmol) in MeCN (5 mL). The reaction mixture was heated at 7O0C for 1.5 h, then partitioned between EtOAc (50 mL) and saturated ammonium chloride solution (50 mL). The aqueous layer was extracted with EtOAc (30 mL). The combined organic layers were dried (sodium sulphate), filtered and concentrated in vacuo. The resulting white solid was dissolved in THF (5 mL) and water (5 niL). Lithium hydroxide (200 mg) was added and the reaction mixture was stirred at r.t. for 2 h, then taken to pH 6 with HCl (2M aqueous). The reaction mixture was concentrated in vacuo, yielding the title compound (0.7 g, 32%) as a white solid. δH (de-DMSO) 8.62 (d, J 2.0 Hz, IH), 8.17 (dd, J8.6, 2.3 Hz, IH), 6.86 (d, J8.3 Hz, IH), 5.00 (q, J9.1 Hz, 2H). LCMS (ES+) 222 (M+H)+.
References:
WO2010/146351,2010,A1 Location in patent:Page/Page column 57-58