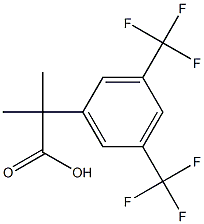
2-(3,5-bis(trifluoroMethyl)phenyl)-2-Methyl propanoic acid synthesis
- Product Name:2-(3,5-bis(trifluoroMethyl)phenyl)-2-Methyl propanoic acid
- CAS Number:289686-70-0
- Molecular formula:C12H10F6O2
- Molecular Weight:300.2
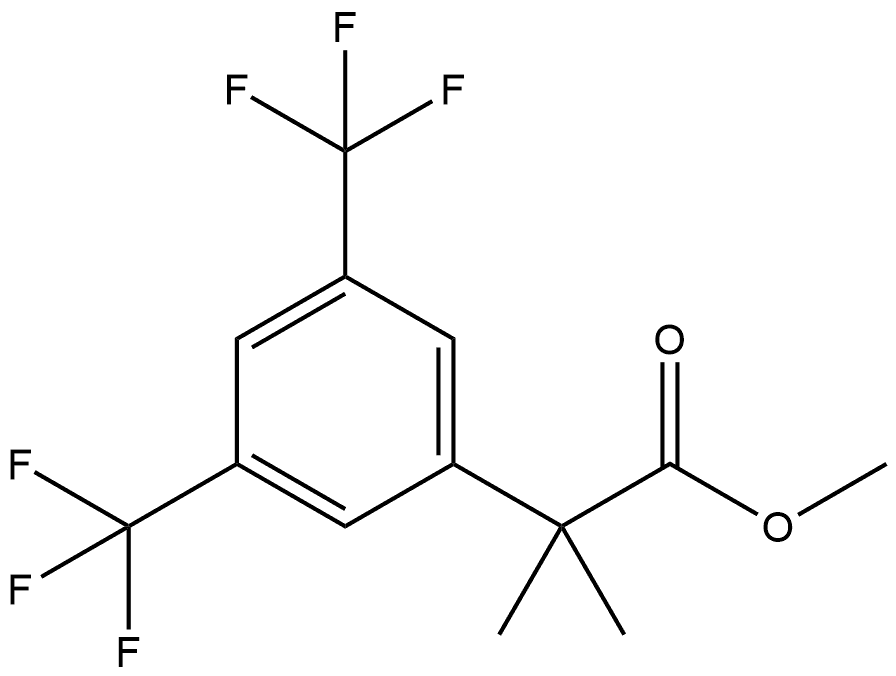
334477-48-4

289686-70-0
The general procedure for the synthesis of 2-(3,5-bis-trifluoromethyl-phenyl)-2-methylpropanoic acid using nerapitant impurity 20 as a starting material was as follows: lithium hydroxide monohydrate (2.13 g, 50.6 mmol) was added to α,α-dimethyl-3,5-bis(trifluoromethyl)benzeneacetic acid methyl ester (5.3 g, 16.88 mmol) in a methanol (60 mL), water (20 mL) and tetrahydrofuran (20 mL) in a suspension. The mixture was degassed and the reaction was stirred at room temperature for 3 days. Upon completion of the reaction, the solvent was removed by distillation under reduced pressure. The residue was suspended in 1 M hydrochloric acid and extracted with ethyl acetate. The organic phases were combined and dried over anhydrous magnesium sulfate, followed by evaporation of the solvent under reduced pressure to afford the target product 2-(3,5-bis-trifluoromethyl-phenyl)-2-methylpropanoic acid as a colorless solid (5.05 g, 100% yield). The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 7.84 (2H, s), 7.80 (1H, s), 1.68 (6H, s).

334477-48-4
2 suppliers
inquiry

289686-70-0
184 suppliers
$8.00/250mg
Yield:289686-70-0 100%
Reaction Conditions:
Stage #1: methyl 2-(3,5-bis(trifluoromethyl)phenyl)-2-methylpropanoatewith lithium hydroxide;water in tetrahydrofuran;methanol at 20; for 72 h;
Stage #2: with hydrogenchloride in water;
Steps:
10 DESCRIPTION 10; α,α-Dimethyl-3,5-bis(trifluoromethyl)benzeneacetic Acid
Lithium hydroxide monohydrate (2.13 g, 50.6 mmol) was added to a suspension of methyl α,α-dimethyl-3,5-bis(trifluoromethyl)benzeneacetate (Description 9, 5.3 g, 16.88 mmol) in methanol (60 mL), water (20 mL) and tetrahydrofuran (20 mL) and the mixture was degassed and stirred at room temperature for 3 days. The solvent was evaporated under reduced pressure and the residue was suspended in hydrochloric acid (1M). The mixture was extracted with ethyl acetate and the combined organic fractions were dried (MgSO4) and the solvent was evaporated under reduced pressure to give the title compound as a colorless solid (5.05 g, 100%). 1H NMR (400 MHz, CDCl3) δ7.84 (2H, s), 7.80 (1H, s), and 1.68 (6H, s).
References:
US2003/225059,2003,A1 Location in patent:Page 22
2052-01-9
272 suppliers
$10.00/1g
73852-19-4
297 suppliers
$5.00/1g

289686-70-0
184 suppliers
$8.00/250mg
201230-82-2
1 suppliers
inquiry
![2-[3,5-Bis(trifluoromethyl)phenyl]propan-2-ol](/CAS/GIF/67570-38-1.gif)
67570-38-1
33 suppliers
$310.00/1g

289686-70-0
184 suppliers
$8.00/250mg
328-72-3
84 suppliers
$11.00/100mg

289686-70-0
184 suppliers
$8.00/250mg