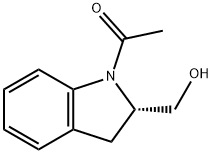
Ethanone, 1-[(2S)-2,3-dihydro-2-(hydroxyMethyl)-1H-indol-1-yl]- synthesis
- Product Name:Ethanone, 1-[(2S)-2,3-dihydro-2-(hydroxyMethyl)-1H-indol-1-yl]-
- CAS Number:297765-25-4
- Molecular formula:C11H13NO2
- Molecular Weight:191.23
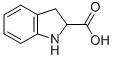
16851-56-2
102 suppliers
inquiry
![Ethanone, 1-[(2S)-2,3-dihydro-2-(hydroxyMethyl)-1H-indol-1-yl]-](/CAS/20150408/GIF/297765-25-4.gif)
297765-25-4
4 suppliers
inquiry
Yield:-
Steps:
Multi-step reaction with 5 steps
1: 91 percent / thionyl chloride / 0 - 20 °C
2: 89 percent / triethylamine; 4-(dimethylamino)pyridine / tetrahydrofuran / 20 - 40 °C
3: 85 percent / DDQ / toluene / 24 h / Heating
4: chiral ferrocene-based complex; triethylamine; hydrogen / [Rh(nbd)2]SbF6 / propan-2-ol / 0.5 h / 100 °C / 76000 Torr
5: 74 percent / lithium borohydride / tetrahydrofuran / 5 h / 20 °C
References:
Kuwano, Ryoichi;Kashiwabara, Manabu;Sato, Koji;Ito, Takashi;Kaneda, Kohei;Ito, Yoshihiko [Tetrahedron Asymmetry,2006,vol. 17,# 4,p. 521 - 535]
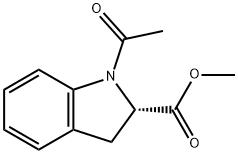
110592-39-7
8 suppliers
inquiry
![Ethanone, 1-[(2S)-2,3-dihydro-2-(hydroxyMethyl)-1H-indol-1-yl]-](/CAS/20150408/GIF/297765-25-4.gif)
297765-25-4
4 suppliers
inquiry
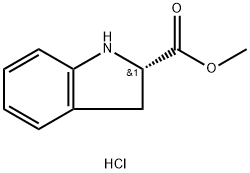
96056-64-3
49 suppliers
$45.00/25mg
![Ethanone, 1-[(2S)-2,3-dihydro-2-(hydroxyMethyl)-1H-indol-1-yl]-](/CAS/20150408/GIF/297765-25-4.gif)
297765-25-4
4 suppliers
inquiry
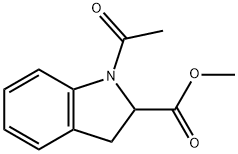
110659-07-9
10 suppliers
inquiry
![Ethanone, 1-[(2S)-2,3-dihydro-2-(hydroxyMethyl)-1H-indol-1-yl]-](/CAS/20150408/GIF/297765-25-4.gif)
297765-25-4
4 suppliers
inquiry

297765-13-0
3 suppliers
inquiry
![Ethanone, 1-[(2S)-2,3-dihydro-2-(hydroxyMethyl)-1H-indol-1-yl]-](/CAS/20150408/GIF/297765-25-4.gif)
297765-25-4
4 suppliers
inquiry