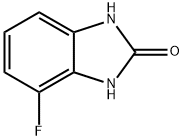
2H-Benzimidazol-2-one,4-fluoro-1,3-dihydro-(9CI) synthesis
- Product Name:2H-Benzimidazol-2-one,4-fluoro-1,3-dihydro-(9CI)
- CAS Number:256519-10-5
- Molecular formula:C7H5FN2O
- Molecular Weight:152.13
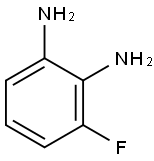
18645-88-0
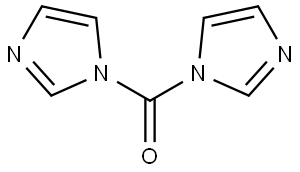
530-62-1

256519-10-5
a) 3-Fluorobenzene-1,2-diamine (0.600 g, 4.76 mmol) was dissolved in tetrahydrofuran (THF, 14.82 mL), and 1,1'-carbonyldiimidazole (0.848 g, 5.23 mmol) was added slowly at room temperature. The reaction mixture was stirred overnight at room temperature and then warmed up to 50 °C to continue the reaction for 24 hours. Upon completion of the reaction, the mixture was cooled to room temperature, a methanol solution of ammonia (1.5 mL) was added and stirring was continued for 30 minutes. Subsequently, the reaction mixture was diluted with water (40 mL), the resulting brown solid was collected by filtration, washed with water and dried under vacuum to afford 4-fluoro-1H-benzo[d]imidazol-2(3H)-one (0.700 g, 97% yield). The product could be used in subsequent steps without further purification.1H NMR (400 MHz, DMSO-d6) δ 6.81 (2H, ddd), 6.88-6.95 (1H, m), 10.82 (1H, s), 11.08 (1H, s); Mass Spectrum (ES-) m/z: 151.19 [M-H]-.

18645-88-0
111 suppliers
$19.00/250mg

530-62-1
969 suppliers
$6.00/25g

256519-10-5
47 suppliers
$69.00/100mg
Yield:256519-10-5 97%
Reaction Conditions:
in tetrahydrofuran at 20 - 50;
Steps:
2.09.a; 2.10.a
a) 3-Fluorobenzene-1,2-diamine (0.600 g, 4.76 mmol) was dissolved in THF (14.82 ml) and 1,1'-carbonyldiimidazole (0.848 g, 5.23 mmol) was added at RT. The reaction mixture was stirred overnight at RT and then heated for 24 hours at 50° C. The mixture was cooled to RT and ammonia in MeOH (1.5 ml) was added and the mixture stirred for 30 minutes. The mixture was dilued with water (40 ml) and the resultant brown solid was collected by filtration, washed with water and then dried in vacuo to afford 4-fluoro-1H-benzo[d]imidazol-2(3H)-one (0.700 g, 97%) which was used in the next step without further purification; 1H NMR (400 MHz, DMSO-d6) 6.81 (2H, ddd), 6.88-6.95 (1H, m), 10.82 (1H, s), 11.08 (1H, s); m/z: (ES-) M-H-, 151.19.
References:
US2011/306613,2011,A1 Location in patent:Page/Page column 31

18645-88-0
111 suppliers
$19.00/250mg
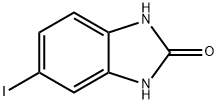
40644-14-2
17 suppliers
$530.00/1g

256519-10-5
47 suppliers
$69.00/100mg