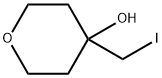
2H-Pyran-4-ol, tetrahydro-4-(iodomethyl)- synthesis
- Product Name:2H-Pyran-4-ol, tetrahydro-4-(iodomethyl)-
- CAS Number:1263505-84-5
- Molecular formula:C6H11IO2
- Molecular Weight:242.05
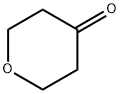
29943-42-8
433 suppliers
$5.00/1g
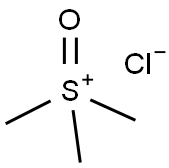
5034-06-0
235 suppliers
$12.00/5g

1263505-84-5
3 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: Tetrahydro-4H-pyran-4-one;trimethyl sulfoxonium chloridewith sodium hydroxide in tetrahydrofuran;water at 50; for 0.5 h;
Stage #2: with hydrogenchloride;sodium iodide in tetrahydrofuran;water at 20; for 19 h;
Steps:
77.1
A mixture of trimethylsulfoxonium chloride (3.08 g, 23.73 mmol) and 2 N aq. sodium hydroxide (11.33 mL, 22.65 mmol) in tetrahydrofuran (13 mL) was heated at 50 oC for 1 h. 2.16 g (21.57 mmol) of tetrahydro-4H-pyran-4-one was added to the mixture and the mixture was stirred at 50 oC for 30 min. After cooling to room temperature, 3.56 g (23.73 mmol) of sodium iodide and 2 N hydrochloric acid (10.8 mL, 21.57 mmol) were added to the mixture. After being stirred at room temperature for 19 h, ethyl acetate was added to the mixture. The mixture was extracted with ethyl acetate three times and the extracts were combined. The extracts were dried over sodium sulfate and concentrated in vacuo to afford a beige solid. The residual solid was recrystallized from acetonitrile to afford 3.13 g (55%) of the title compound.MS (ESI) m/z: 243 (M+H)+.
References:
WO2011/16234,2011,A1 Location in patent:Page/Page column 83