
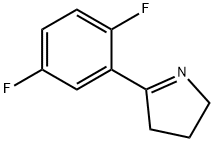
(2R)-2-(2,5-DIFLUOROPHENYL)PYRROLIDINE synthesis
- Product Name:(2R)-2-(2,5-DIFLUOROPHENYL)PYRROLIDINE
- CAS Number:1218935-59-1
- Molecular formula:C10H11F2N
- Molecular Weight:183.2
![Pyrrolidine, 2-(2,5-difluorophenyl)-1-[(S)-(1,1-dimethylethyl)sulfinyl]-, (2R)-](/CAS/20200515/GIF/1443538-31-5.gif)
1443538-31-5

1218935-59-1
GENERAL STEPS: To a solution of (R)-1-((S)-tert-butylsulfinyl)-2-(2,5-difluorophenyl)pyrrolidine (8.60 g, 29.9 mmol) in methanol (60 mL) was slowly added a dioxane solution (37.4 mL, 150 mmol) of 4M hydrochloric acid at 0 °C. The reaction mixture was stirred at room temperature for 12 hours. After completion of the reaction, the mixture was concentrated under vacuum and the residue was dissolved in water (100 mL) and washed with ethyl acetate (100 mL). The aqueous layer was separated and neutralized with 1N aqueous sodium hydroxide (150 mL) followed by extraction with dichloromethane (100 mL × 3). The organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated in vacuum to afford (R)-2-(2,5-difluorophenyl)pyrrolidine (5.06 g, 92% yield) as a slightly reddish oil.1H-NMR (CDCl3, Varian, 400 MHz): δ 1.56-1.65 (1H, m), 1.78-1.93 (3H m), 2.21-2.30 (1H, m), 3.01-3.08 (1H, m), 3.13-3.18 (1H, m), 4.39 (1H, t, J = 7.6 Hz), 6.82-6.88 (1H, m), 6.91-6.97 (1H, m), 7.22-7.26 (1H, m).
1443623-92-4
55 suppliers
inquiry

1218935-59-1
150 suppliers
$115.00/250MG
Yield:1218935-59-1 95%
Reaction Conditions:
with borane-ammonia complex;(R)-Mandelic Acid in water;toluene at 30; for 24 h;Reagent/catalyst;
Steps:
1.4-6.4 4) Preparation of R-2-(2,5-difluorophenyl)pyrrolidine
To the reaction flask, D-mandelic acid (59.6 mg, FW=166, 0.36 mmol), ammonia borane complex (1.19 g, 36 mmol) and toluene (50 mL) were added, stirred for 15 minutes, and the remaining yellow liquid was added Amine (6.5g, 36mmol) was added, then water (0.98mL, 5.42mmol) was added, and the reaction was completed at 30°C for 24 hours. TLC followed the reaction to completion. The reaction solution was concentrated to dryness under reduced pressure, and MTBE and 10 were added to the residue. % Sodium hydroxide solution, stirred, left to separate layers, the organic layer was washed with protective brine, dried over anhydrous sodium sulfate, concentrated to dryness, and the residue was bubble-bubble distilled to obtain 6.24 g (95%) of viscous liquid.
References:
Wuhan University of Technology;Shen Yongcun;Wu Zhouping;Liang Xiayu;Shi Shuai;Xiang Shan;He Letong;Lin Zenghui;Gan Mengqi;Zhang Shixin CN110981779, 2020, A Location in patent:Page/Page column 8-12
![Pyrrolidine, 2-(2,5-difluorophenyl)-1-[(S)-(1,1-dimethylethyl)sulfinyl]-, (2R)-](/CAS/20200515/GIF/1443538-31-5.gif)
1443538-31-5
6 suppliers
inquiry

1218935-59-1
150 suppliers
$115.00/250MG
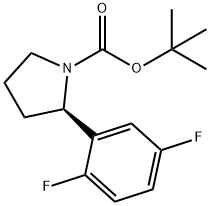
1218935-58-0
8 suppliers
inquiry

1218935-59-1
150 suppliers
$115.00/250MG