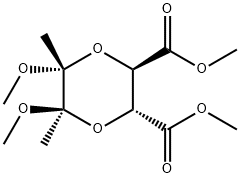
(2R 3R 5R 6R)-DIMETHOXY-5 6-DIMETHYL(1 synthesis
- Product Name:(2R 3R 5R 6R)-DIMETHOXY-5 6-DIMETHYL(1
- CAS Number:181586-74-3
- Molecular formula:C12H20O8
- Molecular Weight:292.28
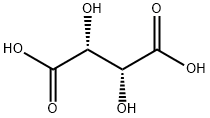
87-69-4
952 suppliers
$5.00/25g
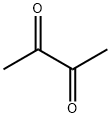
431-03-8
388 suppliers
$21.00/25mL
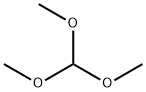
149-73-5
545 suppliers
$12.00/5g

181586-74-3
15 suppliers
$146.72/25G
Yield: 72%
Reaction Conditions:
with toluene-4-sulfonic acid in methanol at 65; for 24 h;
Steps:
Dimethyl (2R,3R,5R,6R)-5,6-dimethoxy-5,6-dimethyl-1,4-dioxane-2,3-dicarboxylate (4)
Using a modification of a procedure reported16 by Maycock and co-workers, a magnetically stirredsolution of L-tartaric acid (11.87 g, 79.09 mmol), 2,3-butanedione (7.25 mL, 80 mmol) and trimethylorthoformate (50 mL, 466 mmol) in dry MeOH (100 mL) was treated with p-toluenesulfonic acidmonohydrate (710 mg, 5 mol%). The resulting solution was heated under reflux for 24 h and the ensuingdeep-red mixture then cooled to room temperature and neutralized with NaHCO3 (4.00 g). Stirring wascontinued for 0.5 h then the reaction mixture was concentrated under reduced pressure. The residue thusobtained was diluted with EtOAc (100 mL) then NaHCO3 (30 mL of a saturated aqueous solution) addedand the separated organic phase washed with NaHCO3 (1 × 30 mL of a saturated aqueous solution) thenwater (1 × 30 mL). The combined aqueous layers were extracted with EtOAc (3 × 30 mL) and thecombined organic phases then washed with brine (2 × 20 mL) before being dried (Na2SO4), filtered andconcentrated under reduced pressure. The yellow solid thus obtained was recrystallized (hexane/EtOAc)and the resulting solid collected and washed successively with ice-cold hexane and ice-cold EtOAc togive bis-ester 416,17 (16.73 g, 72%) as an off-white, crystalline solid, mp 107-108 C (lit.17 mp 106-108 °C), [α]D -135.7 (c 1.1, CHCl3) {lit.17 [α]D -139.6 (c 1.0, CHCl3)} [Found: (M + K)+, 331.0777.C12H20KO8 requires (M + K)+, 331.0795]. 1H NMR (CDCl3, 400 MHz) δH 4.54 (s, 2H), 3.77 (s, 6H), 3.32 (s, 6H), 1.36 (s, 6H); 13C NMR (CDCl3, 100 MHz) δC 168.6, 99.4, 68.9, 52.7, 48.6, 17.5; IR (solid) νmax2992, 1736, 1443, 1377, 1282, 1202, 1139, 1110, 1026, 898, 886, 810, 744 cm-1; MS (ESI, +ve) m/z 315[(M + Na)+, 100%].
References:
Buckler, Joshua N.;Schwartz, Brett D.;Banwell, Martin G. [Heterocycles,2017,vol. 95,# 1,p. 290 - 303]
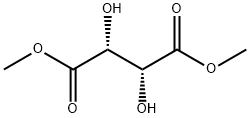
608-68-4
261 suppliers
$14.00/5g

431-03-8
388 suppliers
$21.00/25mL

149-73-5
545 suppliers
$12.00/5g

181586-74-3
15 suppliers
$146.72/25G

87-69-4
952 suppliers
$5.00/25g
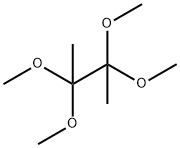
176798-33-7
26 suppliers
$85.00/1 g

181586-74-3
15 suppliers
$146.72/25G

67-56-1
789 suppliers
$7.29/5ml-f

608-68-4
261 suppliers
$14.00/5g

431-03-8
388 suppliers
$21.00/25mL

181586-74-3
15 suppliers
$146.72/25G