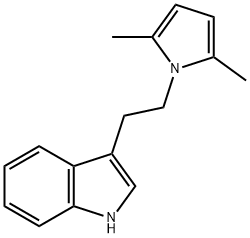
3-[2-(2,5-DIMETHYL-1H-PYRROL-1-YL)ETHYL]-1H-INDOLE synthesis
- Product Name:3-[2-(2,5-DIMETHYL-1H-PYRROL-1-YL)ETHYL]-1H-INDOLE
- CAS Number:95399-28-3
- Molecular formula:C16H18N2
- Molecular Weight:238.33
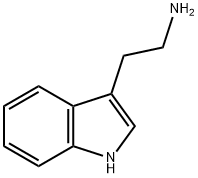
61-54-1
495 suppliers
$12.00/5G
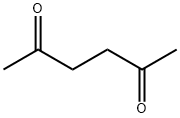
110-13-4
368 suppliers
$10.00/5g
![3-[2-(2,5-DIMETHYL-1H-PYRROL-1-YL)ETHYL]-1H-INDOLE](/CAS/GIF/95399-28-3.gif)
95399-28-3
16 suppliers
$85.00/250mg
Yield:95399-28-3 96%
Reaction Conditions:
with diethylenetriaminepentaacetic acid on magnetic Fe3O4 nanoparticles in ethanol;water at 20; for 0.25 h;Paal-Knorr Pyrrole Synthesis;
Steps:
General Procedure for Synthesis of Pyrroles in Presence of Fe3O4(at)DTPA
General procedure: To a solution of amine (1 mmol) and hexan-2,5-dione (1 mmol) in EtOH-H2O (2 ml, 1:1) at room temperature was added catalyst (40 mg ~4 mol%). The mixture was stirred at this temperature for the required period of time (Table 3). The reaction was monitored using TLC (silica gel, 3:1 n-hexane-acetone). After completion of the reaction, 10 ml of ethanol was added, and the catalyst was removed using an external magnet. Concentration gave the crude product as a solid. The crude product was purified by recrystallization from ethanol to afford pure product. For the compounds that were oils (3h, 3m, 3o), identification was made by matching 1H-NMR and 13C-NMR values withthose in the literature.37,40
References:
Hemmati, Saba;Mohammadi, Pourya;Sedrpoushan, Alireza;Maleki, Behrooz [Organic Preparations and Procedures International,2018,vol. 50,# 5,p. 465 - 481]

61-54-1
495 suppliers
$12.00/5G
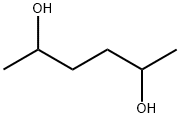
2935-44-6
140 suppliers
$14.00/1g
![3-[2-(2,5-DIMETHYL-1H-PYRROL-1-YL)ETHYL]-1H-INDOLE](/CAS/GIF/95399-28-3.gif)
95399-28-3
16 suppliers
$85.00/250mg
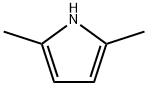
625-84-3
194 suppliers
$17.67/5gm:

61-54-1
495 suppliers
$12.00/5G
![3-[2-(2,5-DIMETHYL-1H-PYRROL-1-YL)ETHYL]-1H-INDOLE](/CAS/GIF/95399-28-3.gif)
95399-28-3
16 suppliers
$85.00/250mg
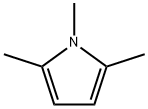
930-87-0
68 suppliers
$44.00/5mL
![3-[2-(2,5-DIMETHYL-1H-PYRROL-1-YL)ETHYL]-1H-INDOLE](/CAS/GIF/95399-28-3.gif)
95399-28-3
16 suppliers
$85.00/250mg