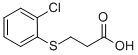
3-(2-CHLORO-PHENYLSULFANYL)-PROPIONIC ACID synthesis
- Product Name:3-(2-CHLORO-PHENYLSULFANYL)-PROPIONIC ACID
- CAS Number:99585-16-7
- Molecular formula:C9H9ClO2S
- Molecular Weight:0
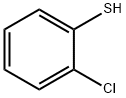
6320-03-2
169 suppliers
$12.00/1g
539-74-2
293 suppliers
$5.00/5g

99585-16-7
15 suppliers
$65.00/50mg
Yield:99585-16-7 93%
Reaction Conditions:
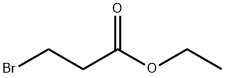
Stage #1: 2-chlorothiphenol;Ethyl 3-bromopropionatewith potassium hydroxide;ethanol;water at 20; for 64 h;
Stage #2: with hydrogenchloride;water pH=1;
Steps:
44
Example 44 - Preparation of 3-(2-Chloro-phenylsulfanyl)-propionic acid (Compound 44); [137] To a 500 mL round bottom flask, equipped with a magnetic stir bar, was added 2- chlorobenzenethiol (2.00 mL, 18 mmol), ethyl 3-bromopropionate (2.26 mL, 18 mmol), potassium hydroxide (2.08 g, 37 mmol), and 50 mL ethyl alcohol. The reaction mixture was stirred at room temperature for 4 hours, 15 mL water was added and the mixture was stirred for 42 hours at room temperature. Ethyl 3-bromopropionate (1.70 mL, 13 mmol) and potassium hydroxide (2.19 g, 39 mmol) were added in three aliquots during the next 18 hours. Solvent was removed under reduced pressure. Residual was dissolved in 150 mL water and acidified with aqueous 1 N hydrochloric acid solution to pH 1. Filtration yielded the product (3.56 g, 93%) as a white solid. IH NMR (d6-DMSO): δ 12.41, s, IH (COOH); δ 7.46, dd, IH (aryl H); δ 7.40, dd, IH (aryl H); δ 7.34, dt, IH (aryl H); δ 7.20, dt, IH (aryl H); 5 3.18, t, 2H (CH2 α to S); δ 2.59, t, 2H (CH2 α to COOH).
References:
WO2008/14430,2008,A1 Location in patent:Page/Page column 52
107-94-8
225 suppliers
$9.00/1g

6320-03-2
169 suppliers
$12.00/1g

99585-16-7
15 suppliers
$65.00/50mg
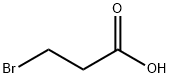
590-92-1
242 suppliers
$10.00/1g

6320-03-2
169 suppliers
$12.00/1g

99585-16-7
15 suppliers
$65.00/50mg

6320-03-2
169 suppliers
$12.00/1g
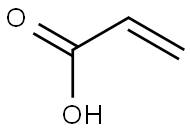
79-10-7
737 suppliers
$20.50/500ml

99585-16-7
15 suppliers
$65.00/50mg

6320-03-2
169 suppliers
$12.00/1g

99585-16-7
15 suppliers
$65.00/50mg