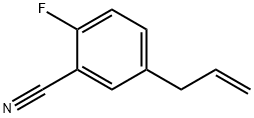
3-(3-CYANO-4-FLUOROPHENYL)-1-PROPENE synthesis
- Product Name:3-(3-CYANO-4-FLUOROPHENYL)-1-PROPENE
- CAS Number:943247-49-2
- Molecular formula:C10H8FN
- Molecular Weight:161.18
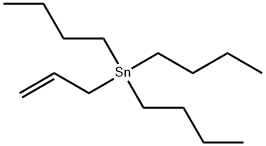
24850-33-7
197 suppliers
$12.00/5g
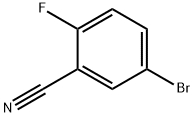
179897-89-3
317 suppliers
$10.00/5g

943247-49-2
8 suppliers
inquiry
Yield:943247-49-2 61%
Reaction Conditions:
tetrakis(triphenylphosphine) palladium(0) in N,N-dimethyl-formamide at 20 - 50;
Steps:
27
Example 27: 5-Propyl-N-l,3-thiazol-2-yl-lH-indazol-3-amineH2, Pd/C, EtOH[0333] To a stirred solution of 5-bromo-2-fluorobenzonitrile (1.50 g, 7.50 mmol) in DMF (20 mL) were added allyl tributyltin (3.73 g, 11.25 mmol) and tetrakis(triphenylphosphine)palladium (433 mg, 0.375 mmol) under nitrogen atmosphere at room temperature. The mixture was stirred at 500C overnight. After cooling, the mixture was diluted with EtOAc, washed with IN HCl, H2O, and brine, dried (MgSO4), filtered, and concentrated in vacuo. The crude oil was passed through a short NH-SiO2 column, and the filtrate was concentrated in vacuo. Purification by silica gel chromatography (hexane/EtOAc=20: 1 to 10:1) gave 739 mg (61 %) of 5-allyl-2-fluorobenzonitrile (compound 27A) as a colorless oil. 1H NMR (300 MHz, CDCl3) δ 3.39 (d, 2H, 7=6.59 Hz) 5.02 - 5.23 (m, 2H) 5.79 - 6.01 (m, IH) 7.14 (t, IH, 7=8.57 Hz) 7.35 - 7.50 (m, 2H) [0334] To a stirred solution of 5-allyl-2-fluorobenzonitrile (0.74 g, 4.57 mmol) in n-BuOH (30 mL) was added hydrazine monohydrate (0.67 mL, 13.8 mmol). The mixture was stirred under reflux overnight, and concentrated in vacuo. The residue was diluted with EtOAc, washed with H2O and brine, dried (MgSO4), filtered, and concentrated in vacuo. The precipitate was collected, and washed with hexane to give the mixture of 5-allyl-lH-indazol- 3-arnine (compound 27B) and 5-propyl-lH-indazol-3-amine (compound 27C) (2:5). The
References:
WO2007/75847,2007,A2 Location in patent:Page/Page column 108-109