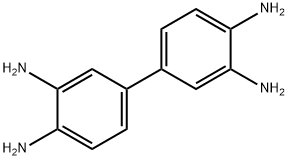
3,3'-Diaminobenzidine synthesis
- Product Name:3,3'-Diaminobenzidine
- CAS Number:91-95-2
- Molecular formula:C12H14N4
- Molecular Weight:214.27
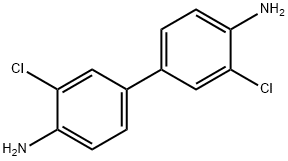
91-94-1
101 suppliers
$30.00/1g

91-95-2
386 suppliers
$6.00/1g
Yield:91-95-2 93.1%
Reaction Conditions:
with ammonium hydroxide;tetrabutyl-ammonium chloride;ammonia;copper dichloride at 10 - 180; under 20252 - 24002.4 Torr; for 7.5 h;Inert atmosphere;Autoclave;Reagent/catalyst;Temperature;Pressure;
Steps:
1-3 Example one
Add 173.0g (equivalent to 0.53mol) of dichlorodiphenyldiamine and 500g of 25% ammonia in the beating kettle cooled with ice water. After beating, add 0.4g of cuprous chloride,In an autoclave containing 5.2 g of proline and 5.2 g of tetrabutylammonium chloride, the air in the autoclave was replaced with nitrogen four times, and then the beating kettle was washed with 248 g of 25% ammonia water and transferred to the autoclave. The temperature of the system is controlled to not exceed 10 °C during the whole process of beating, transferring, replacing and washing. The agitation of the autoclave was turned on and 170 g of ammonia gas was continuously passed into the autoclave. The autoclave was closed and the temperature was raised. After about 1.5h, the reaction system in the autoclave was heated to 170 °C and the pressure was 2.7 MPa, and the reaction was kept at this temperature and pressure for 3 h. After that, the temperature is continued to increase, and after about 0.5h, the temperature is increased to 180 °C and the pressure is 3.2 MPa. The reaction is kept at this temperature and pressure for 3 h to complete the amino substitution reaction. The heating was stopped, and the temperature of the autoclave was lowered to 130 °C at room temperature, and the pressure dropped to 1.5-2.0 MPa. At this time, ammonia discharge begins to cool down. When the temperature of the autoclave is lowered to about 70-80 °C, it is blown back with nitrogen and discharged into the cooling autoclave. After the material liquid is continuously cooled to 10-15 °C under the protection of nitrogen, pressure filtration is performed. The filter cake was washed three times with 150 g of 25 % ammonia water, and then washed three times with 130 g of water to obtain about 140 g of crude 3,3',4,4'-tetraaminobiphenyl. The whole washing process was protected by nitrogen. Put the obtained crude product of 3,3',4,4'-tetraaminobiphenyl into a decolorization kettle, add 3500g methanol and 8.0g activated carbon, heat up to reflux under the protection of nitrogen replacement, and perform decolorization treatment for about 1 h. Subsequently, press filtration with a sand core funnel at a high temperature, and the filter cake was washed with 100 g of hot water. After the filtrate is combined, the temperature of the filtrate is lowered to 10-15 °C to crystallize 3,3',4,4'-tetraaminobiphenyl. After precipitation of crystals, suction filtration was performed, and the filter cake was vacuum dried at 70 °C for 5 hours to obtain 107.8 g of 3,3',4,4'-tetraaminobiphenyl solid product. It was determined that the 3,3',4,4'-tetraaminobiphenyl content (namely product purity) of the product was 98.0%, and the reaction yield was 93.1%.
References:
CN112920054,2021,A Location in patent:Paragraph 0061-0076
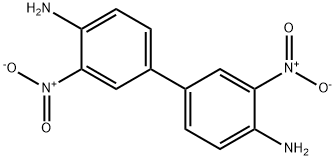
6271-79-0
80 suppliers
$28.00/1g

91-95-2
386 suppliers
$6.00/1g
![[1,1'-Biphenyl]-3,3'-diamine, 4,4'-dinitro-](/CAS/20210111/GIF/74959-03-8.gif)
74959-03-8
0 suppliers
inquiry

91-95-2
386 suppliers
$6.00/1g
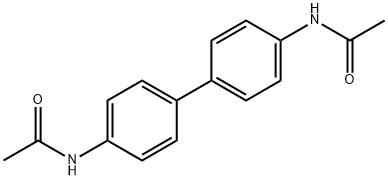
613-35-4
41 suppliers
$20.00/10mg

91-95-2
386 suppliers
$6.00/1g
6378-90-1
15 suppliers
inquiry

91-95-2
386 suppliers
$6.00/1g