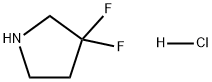
3,3-DIFLUOROPYRROLIDINE HYDROCHLORIDE synthesis
- Product Name:3,3-DIFLUOROPYRROLIDINE HYDROCHLORIDE
- CAS Number:163457-23-6
- Molecular formula:C4H7F2N.ClH
- Molecular Weight:143.56
195447-25-7

163457-23-6
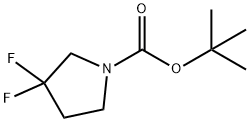
Part C: 1,1-dimethylethyl 3,3-difluoropyrrolidine-1-carboxylate (0.868 g, 4.19 mmol) was dissolved in 1.5 mL of 1,4-dioxane and cooled to 0°C. A 1,4-dioxane solution (11 mL, 44 mmol) of 4 M hydrogen chloride was slowly added with stirring. The reaction mixture was continued to be stirred at 0°C for 40 minutes, then brought to room temperature and stirred for 1 hour. Upon completion of the reaction, the solvent was removed by concentration under reduced pressure to afford 0.65 g of 3,3-difluoropyrrolidine hydrochloride (100% yield). The product was characterized by 1H-NMR (CD3OD): δ 3.54 (2H, t, J = 11.9 Hz), 3.43 (2H, t, J = 7.8 Hz), 2.40 (2H, m).

195447-25-7
75 suppliers
$45.00/250mg

163457-23-6
283 suppliers
$15.00/250mg
Yield:163457-23-6 100%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane at 0 - 20; for 1.66667 h;
Steps:
431.C
[0358] Part C: To a solution of 1,1-dimethylethyl 3,3-difluoropyrrolidine-1-carboxylate (0.868 g, 4.19 mmol) in 1.5 mL of 1,4-dioxane was added a solution of hydrogen chloride in 1,4-dioxane (4 M, 11 mL, 44 mmol) at 0° C. The mixture was stirred at 0° C. for 40 min, at room temperature for 1 h and was then concentrated to afford 0.65 g (100%) of 3,3-difluoropyrrolidine hydrochloride: 1H-NMR (CD3OD) δ 3.54 (2H, t, J=11.9 Hz), 3.43 (2H, t, J=7.8 Hz), 2.40 (2H, m).
References:
O'Connor, Stephen P.;Lawrence, Michael;Shi, Yan;Stein, Philip D. US2004/186134, 2004, A1 Location in patent:Page 241