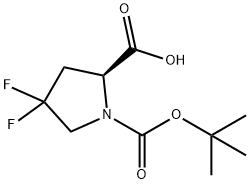
N-BOC-4,4-difluoro-L-proline synthesis
- Product Name:N-BOC-4,4-difluoro-L-proline
- CAS Number:203866-15-3
- Molecular formula:C10H15F2NO4
- Molecular Weight:251.23
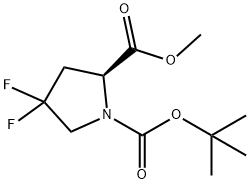
203866-17-5

203866-15-3
The general procedure for the synthesis of N-Boc-4,4-difluoro-L-proline from N-Boc-4,4-difluoro-L-proline methyl ester was as follows: 1. 1.08 g (4.08 mmol) of tert-butyl (S)-2-methyl-4-pyrrolidine-1,4-difluoropyrrolidine-2-carboxylate (purchased from Aldrich, Catalog No. 702463) was dissolved in a mixed solvent of 10 mL of methanol and 10 mL of tetrahydrofuran. 2. 6.12 mL of 2 M aqueous sodium hydroxide was added to the above solution. 3. The reaction mixture was stirred at room temperature for 1 hour. 4. Upon completion of the reaction, the organic solvent was removed under reduced pressure. 5. The remaining aqueous phase was diluted with an appropriate amount of water and then acidified by adding 6.12 mL of 2 M hydrochloric acid. 6. After acidification, the product was extracted with dichloromethane (3 times) and the organic layers were combined. 7. The organic layer was washed with brine, dried with anhydrous magnesium sulfate, filtered and the solvent was removed under reduced pressure. 8. 1.01 g (99% yield) of N-Boc-4,4-difluoro-L-proline was finally obtained as a white solid.LRMS (m/z): 250 (M-1)+.

203866-17-5
146 suppliers
$13.00/100mg

203866-15-3
186 suppliers
$15.00/250mg
Yield:203866-15-3 99%
Reaction Conditions:
Stage #1: 1-tert-butyl 2-methyl (2S)-4,4-difluoropyrrolidine-1,2-dicarboxylatewith water;sodium hydroxide in tetrahydrofuran;methanol at 20; for 1 h;
Stage #2: with hydrogenchloride in water;
Steps:
63
PREPARATION 63CS^-l -iiert-ButoxycarbonylJ^^-difluoropyrrolidine^-carboxylic acid1.08 g (4.08 mmol) of (S)^ -ferf-butyl 2-methyl 4,4-difluoropyrrolidine-1 ,2-dicarboxylate (purchased from Aldrich; cat. no. 702463) were dissolved in a mixture of 10 mL of methanol and 10 mL of tetrahydrofurane and 6.12 mL of a 2M aqueous solution of sodium hydroxide were added. The solution was stirred at room temperature for 1 hour and then the organic solvents were removed. The remaining solution was diluted with water and 6.12 mL of 2M hydrochloric acid were added. The product that precipitated was extracted with dichloromethane (x3), the combined organic layers were washed with brine, dried over magnesium sulphate, filtered and the solvent was removed. 1 .01 g (99% yield) of the title compound were obtained as a white solid.LRMS (m/z): 250 (M-1 )+.
References:
WO2012/146666,2012,A1 Location in patent:Page/Page column 138-139
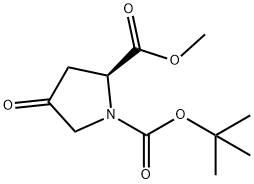
102195-80-2
246 suppliers
$5.00/250mg

203866-15-3
186 suppliers
$15.00/250mg
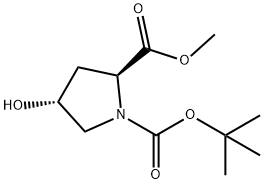
74844-91-0
418 suppliers
$5.00/5g

203866-15-3
186 suppliers
$15.00/250mg

24424-99-5
870 suppliers
$13.50/25G

203866-15-3
186 suppliers
$15.00/250mg
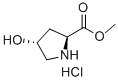
40216-83-9
335 suppliers
$6.00/5g

203866-15-3
186 suppliers
$15.00/250mg