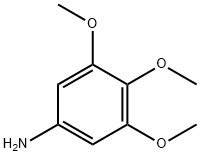
3,4,5-Trimethoxyaniline synthesis
- Product Name:3,4,5-Trimethoxyaniline
- CAS Number:24313-88-0
- Molecular formula:C9H13NO3
- Molecular Weight:183.2
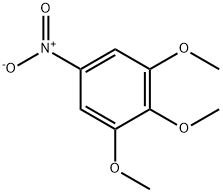
6307-90-0

24313-88-0
Step B / Intermediate B9: Synthesis of 3,4,5-trimethoxyaniline To a solution of 1,2,3-trimethoxy-5-nitrobenzene (6.64 g, 31.2 mmol) in ethanol (250 mL) was added palladium carbon (10%, 300 mg) and hydrazine hydrate (85%, 5.7 mL). The reaction mixture was heated to reflux for 1 hour after gas release ceased. Upon completion of the reaction, the mixture was cooled to room temperature and the catalyst was removed by filtration followed by evaporation of the solvent to afford 3,4,5-trimethoxyaniline (5.5 g, 96.4% yield) as a white solid. The product was characterized by 1H NMR (400 MHz, DMSO-d6): δ 5.86 (s, 2H), 4.82 (br, 2H), 3.64 (s, 6H), 3.50 (s, 3H).

6307-90-0
39 suppliers
inquiry

24313-88-0
290 suppliers
$10.00/1g
Yield:24313-88-0 96.4%
Reaction Conditions:
with hydrazine hydrate;palladium 10% on activated carbon in ethanol; for 1 h;Reflux;
Steps:
B9.B
Step B / Intermediate B9: 3,4,5-trimethoxyani.ineTo a solution of 1 ,2,3-trimethoxy-5-nifrobenzene (6.64 g, 31.2 mrnol ) in ethanol (250 mL) was added palladium on carbon (10 %, 300 mg) and hydrazine hydrate (85 %, 5.7 mL). After emission of gas has ceased, the reaction mixture was heated at refluxed for 1 hour, cooling to room temperature, filtered and evaporated to afford the product 3,4,5-trimethoxyaniline as a white solid (5.5 g, yield 96.4 %). H NMR (400 MHz, DMSO-d6) δ ppm 5.86 (s, 2H), 4.82 (br, 2H), 3.64 (s, 6H), 3.50 (s, 3H).
References:
WO2012/92880,2012,A1 Location in patent:Page/Page column 60

115483-29-9
2 suppliers
inquiry

24313-88-0
290 suppliers
$10.00/1g
2675-79-8
232 suppliers
$8.00/1g

24313-88-0
290 suppliers
$10.00/1g
3086-62-2
135 suppliers
$42.29/25g

24313-88-0
290 suppliers
$10.00/1g
25245-29-8
78 suppliers
$16.00/100mg

24313-88-0
290 suppliers
$10.00/1g